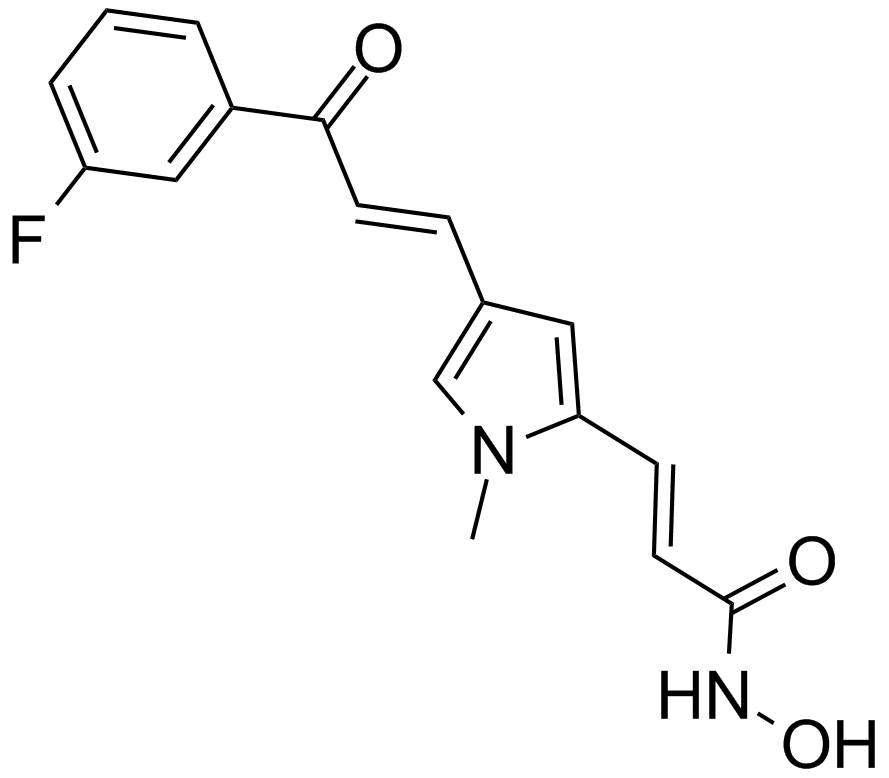
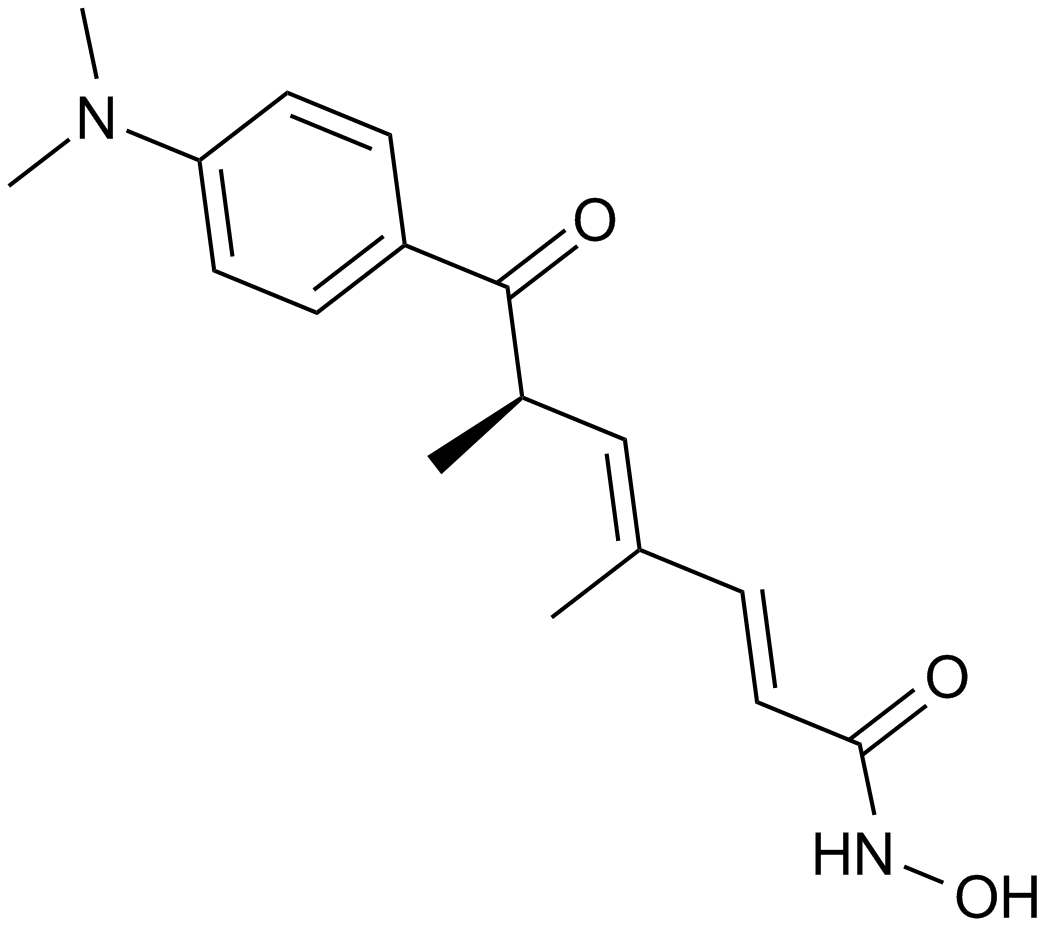
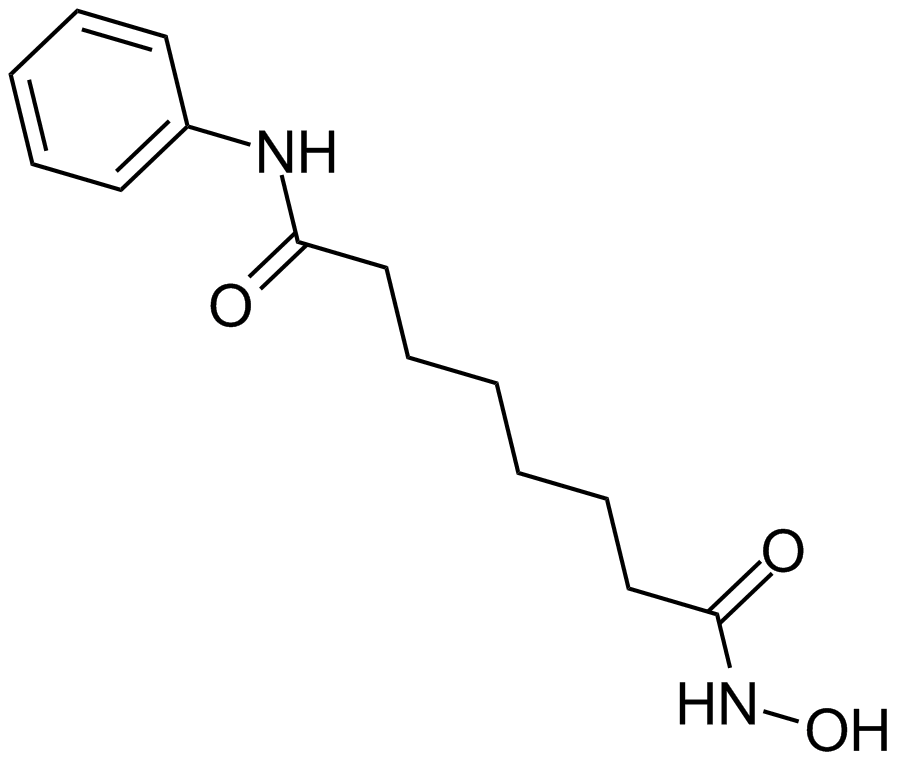
MC1568
MC1568, a derivative of (Aryloxopropenyl)pyrrolyl hydroxyamide, is a novel, potent and specific inhibitor of class II histone deacetylase (HDAC), including two subclasses IIa (HDAC4, HDAC5, HDAC6, HDAC7 and HDAC9) and IIb (HDAC6 and HDAC 10), that exhibits strong inhibition against maize class II HDAC with 50% inhibition concentration IC50 value of 22 μM. MC1568 has been found to tissue-selectively inhibits HDAC and arrest myogenesis in cultured muscle cells through three possible mechanisms, including decreasing the expression of myocyte enhancer factor 2D, stabilizing the HDAC-HDAC3-MEF2D complex and inhibiting the acetylation of differentiation-induced MEF2D. Moreover, MC1568 is able to interfere with RAR- and PPARγ-mediated differentiation-inducing signaling pathways.
Reference
Mai A, Massa S, Pezzi R, Simeoni S, Rotili D, Nebbioso A, Scognamiglio A, Altucci L, Loidl P, Brosch G. Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamides. J Med Chem. 2005 May 5;48(9):3344-53.
Nebbioso A, Dell'Aversana C, Bugge A, Sarno R, Valente S, Rotili D, Manzo F, Teti D, Mandrup S, Ciana P, Maggi A, Mai A, Gronemeyer H, Altucci L. HDACs class II-selective inhibition alters nuclear receptor-dependent differentiation. J Mol Endocrinol. 2010 Oct;45(4):219-28. doi: 10.1677/JME-10-0043. Epub 2010 Jul 16.
Nebbioso A, Manzo F, Miceli M, Conte M, Manente L, Baldi A, De Luca A, Rotili D, Valente S, Mai A, Usiello A, Gronemeyer H, Altucci L. Selective class II HDAC inhibitors impair myogenesis by modulating the stability and activity of HDAC-MEF2 complexes. EMBO Rep. 2009 Jul;10(7):776-82. doi: 10.1038/embor.2009.88. Epub 2009 Jun 5.
- 1. Hari Prasad, Rajini Rao. "The Amyloid Clearance Defect in ApoE4 Astrocytes is Corrected by Epigenetic Restoration of NHE6." bioRxiv. 2018.January. 4
- 2. Griffin EA Jr, Melas PA, et al. "Prior alcohol use enhances vulnerability to compulsive cocaine self-administration by promoting degradation of HDAC4 and HDAC5." Sci Adv. 2017 Nov 1;3(11):e1701682. PMID:29109977
- 3. Ha, Soon-Duck, et al. "Inhibition of IL-1β Expression by Anthrax Lethal Toxin is Reversed by HDAC8 Inhibition in Murine Macrophages." Journal of Biological Chemistry (2016): jbc-M115. PMID:26912657
| Storage | Store at -20°C |
| M.Wt | 314.31 |
| Cas No. | 852475-26-4 |
| Formula | C17H15FN2O3 |
| Synonyms | MC 1568, MC-1568 |
| Solubility | insoluble in EtOH; insoluble in H2O; ≥15.7 mg/mL in DMSO |
| Chemical Name | (E)-3-[4-[(E)-3-(3-fluorophenyl)-3-oxoprop-1-enyl]-1-methylpyrrol-2-yl]-N-hydroxyprop-2-enamide |
| SDF | Download SDF |
| Canonical SMILES | C[n]1c(C=CC(NO)=O)cc(C=CC(c2cccc(F)c2)=O)c1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Maize HD2, HD1-B, and HD1-A enzyme inhibition |
The enzyme liberated tritiated acetic acid from the substrate, which was quantified by scintillation counting. IC50 values are results of triple determinations. A 50 μL sample of maize enzyme (at 30 °C) was incubated (30 mins) with 10 μL of total [3H]acetate-prelabeled chicken reticulocyte histones (2 mg/mL). Reaction was stopped by addition of 50 μL of 1 M HCl/0.4 M acetate and 800 μL of ethyl acetate. After centrifugation (10000 g, 5 mins), an aliquot of 600 μL of the upper phase was counted for radioactivity in 3 mL of liquid scintillation cocktail. MC1568 was tested at a starting concentration of 40 μM, and active substances were diluted further. NaB, VPA, TSA, SAHA, 85TPX, HC-toxin, and tubacin were used as the reference compounds, and blank solvents were used as negative controls. |
| Cell experiment [2]: | |
|
Cell lines |
3T3-L1 cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20 °C for several months. |
|
Reaction Conditions |
~ 10 μM; 8 days |
|
Applications |
In 3T3-L1 cells, MC1568 attenuated PPARγ-induced adipogenesis. |
| Animal experiment [2]: | |
|
Animal models |
PPRE-Luc transgenic C57BL/6 mice |
|
Dosage form |
50 mg/kg; p.o.; q.d., for 7 days |
|
Applications |
In PPRE-Luc transgenic C57BL/6 mice, MC1568 (50 mg/kg) impaired PPARγ signaling mostly in the heart and adipose tissues. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Mai A, Massa S, Pezzi R, Simeoni S, Rotili D, Nebbioso A, Scognamiglio A, Altucci L, Loidl P, Brosch G. Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamides. J Med Chem. 2005 May 5;48(9):3344-53. [2]. Nebbioso A, Dell'Aversana C, Bugge A, Sarno R, Valente S, Rotili D, Manzo F, Teti D, Mandrup S, Ciana P, Maggi A, Mai A, Gronemeyer H, Altucci L. HDACs class II-selective inhibition alters nuclear receptor-dependent differentiation. J Mol Endocrinol. 2010 Oct;45(4):219-28. |
|
| Description | MC1568 is a selective inhibitor of HDAC for maize HD1-A with IC50 of 100 nM. It is 34-fold more selective for HD1-A than HD1-B. | |||||
| Targets | HD1-A (Maize) | HD1-B (Maize) | ||||
| IC50 | 100 nM | 3400 nM | ||||
Quality Control & MSDS
- View current batch:
Chemical structure

Related Biological Data
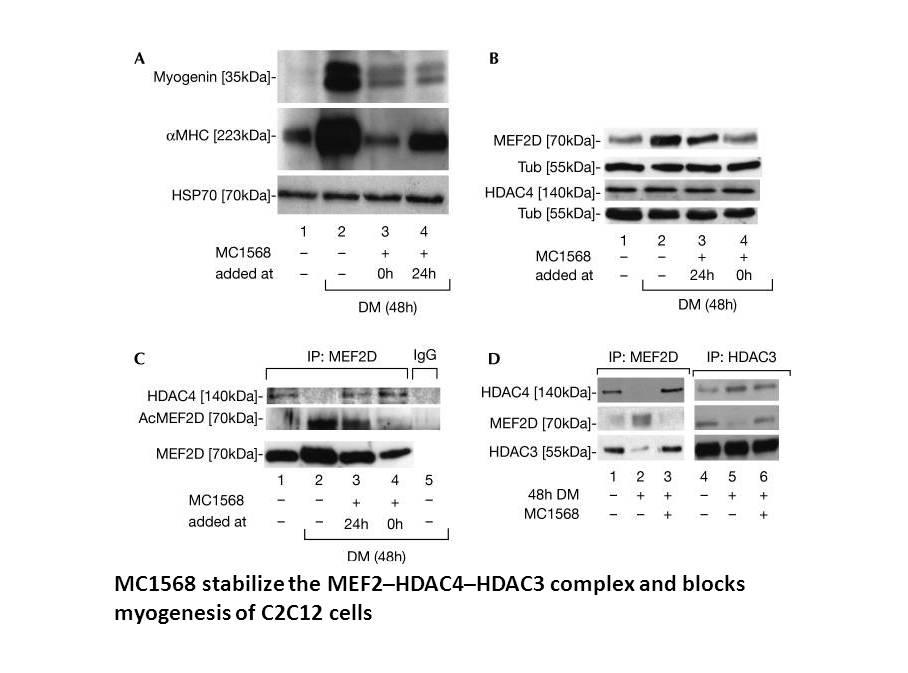
Related Biological Data
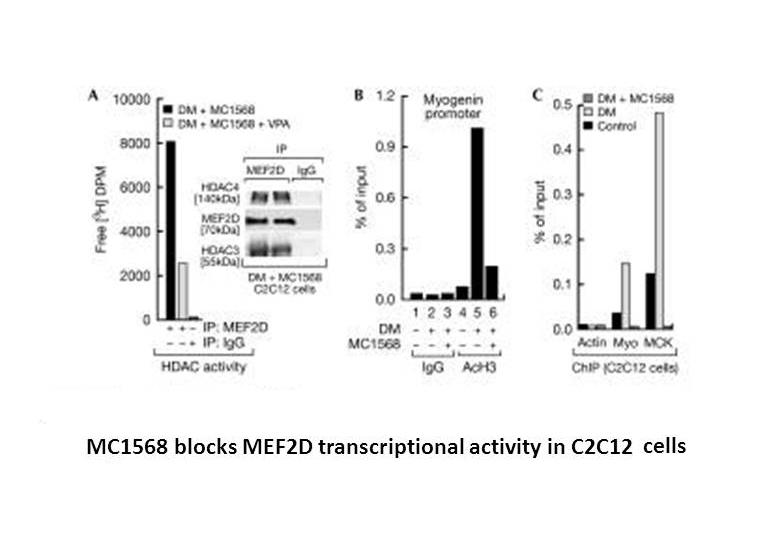
Related Biological Data

Related Biological Data