Search results for: 'signaling pathways proteases serine protease'
-
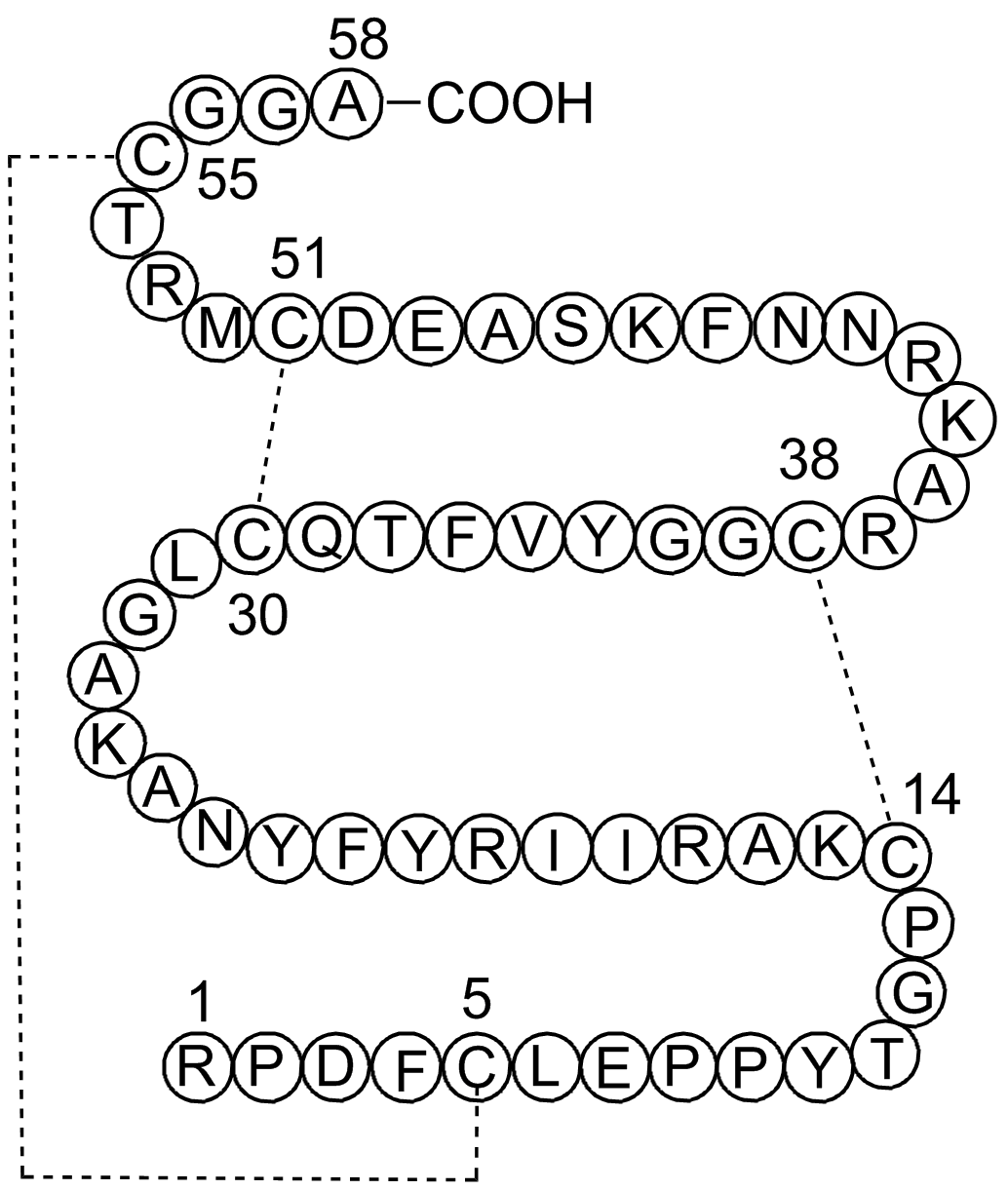 A2574 Aprotinin8 CitationTarget: Trypsin|Chymotrypsin|Kallikrein|TrypsinogenSummary: serine protease inhibitor
A2574 Aprotinin8 CitationTarget: Trypsin|Chymotrypsin|Kallikrein|TrypsinogenSummary: serine protease inhibitor -
 P1565 Recombinant Human Mammary Serine Protease Inhibitor
P1565 Recombinant Human Mammary Serine Protease Inhibitor -
 MA5518 Anti-Transmembrane Protease Serine 2 Rabbit Monoclonal AntibodySummary: Anti-Transmembrane Protease Serine 2 Rabbit Monoclonal Antibody
MA5518 Anti-Transmembrane Protease Serine 2 Rabbit Monoclonal AntibodySummary: Anti-Transmembrane Protease Serine 2 Rabbit Monoclonal Antibody -
 K1103 SUMO ProteaseSummary: SUMO Protease
K1103 SUMO ProteaseSummary: SUMO Protease -
 K1098 TEV ProteaseSummary: TEV Protease
K1098 TEV ProteaseSummary: TEV Protease -
 L1044 DiscoveryProbe™ NF-κB Signaling LibrarySummary: A unique collection of 73 NF-κB inhibitors for NF-κB signaling pathway research.
L1044 DiscoveryProbe™ NF-κB Signaling LibrarySummary: A unique collection of 73 NF-κB inhibitors for NF-κB signaling pathway research. -
 L1026 DiscoveryProbe™ Neuronal Signaling Library1 CitationSummary: A unique collection of 556 neuronal signaling-related small molecules for neuroscience reasearch.
L1026 DiscoveryProbe™ Neuronal Signaling Library1 CitationSummary: A unique collection of 556 neuronal signaling-related small molecules for neuroscience reasearch. -
 K1101 PreScission Protease (PSP)9 CitationSummary: HRV 3C protease
K1101 PreScission Protease (PSP)9 CitationSummary: HRV 3C protease -
 K3201 IdeZ Protease (IgG-specific)Summary: Epidemic globulin-specific IdeZ protease with His tag
K3201 IdeZ Protease (IgG-specific)Summary: Epidemic globulin-specific IdeZ protease with His tag -
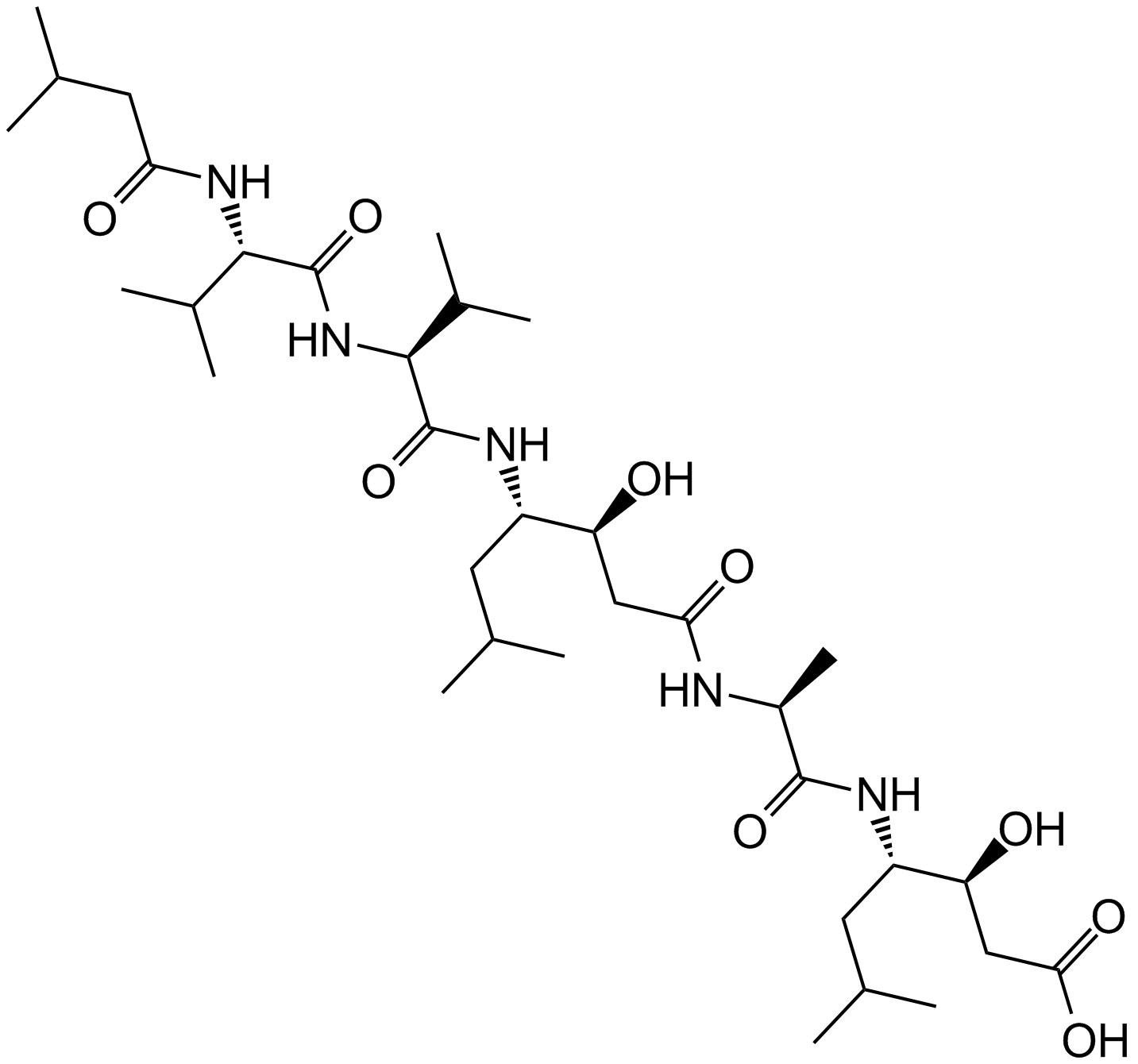 A2571 Pepstatin A9 CitationTarget: Cathepsins|Renin|HIV proteases|PepsinsSummary: aspartic proteases inhibitor
A2571 Pepstatin A9 CitationTarget: Cathepsins|Renin|HIV proteases|PepsinsSummary: aspartic proteases inhibitor


