Serine Protease
Serine proteases, named for the nucleophilic Ser residue at the active site, are a large and diverse group of proteases (accounting for approximately one-third of all proteases) that are characterized by the presence of the Asp-His-Ser “charge relay” system (catalytic triad) in their chemical structures. However, with the development of technology, a variety of novel serine proteases with catalytic triads and dyads have been discovered, including Ser-His-Glu, Ser-Lys/His, His-Ser-His and N-terminal Ser. Serine proteases are traditionally divided into four clans based on the Asp-His-Ser triad in different structural contexts, including chymotrypsin-like proteases, subtilisin-like proteases, carboxypeptidase Y-like proteases and Clp protease-like proteases.
-
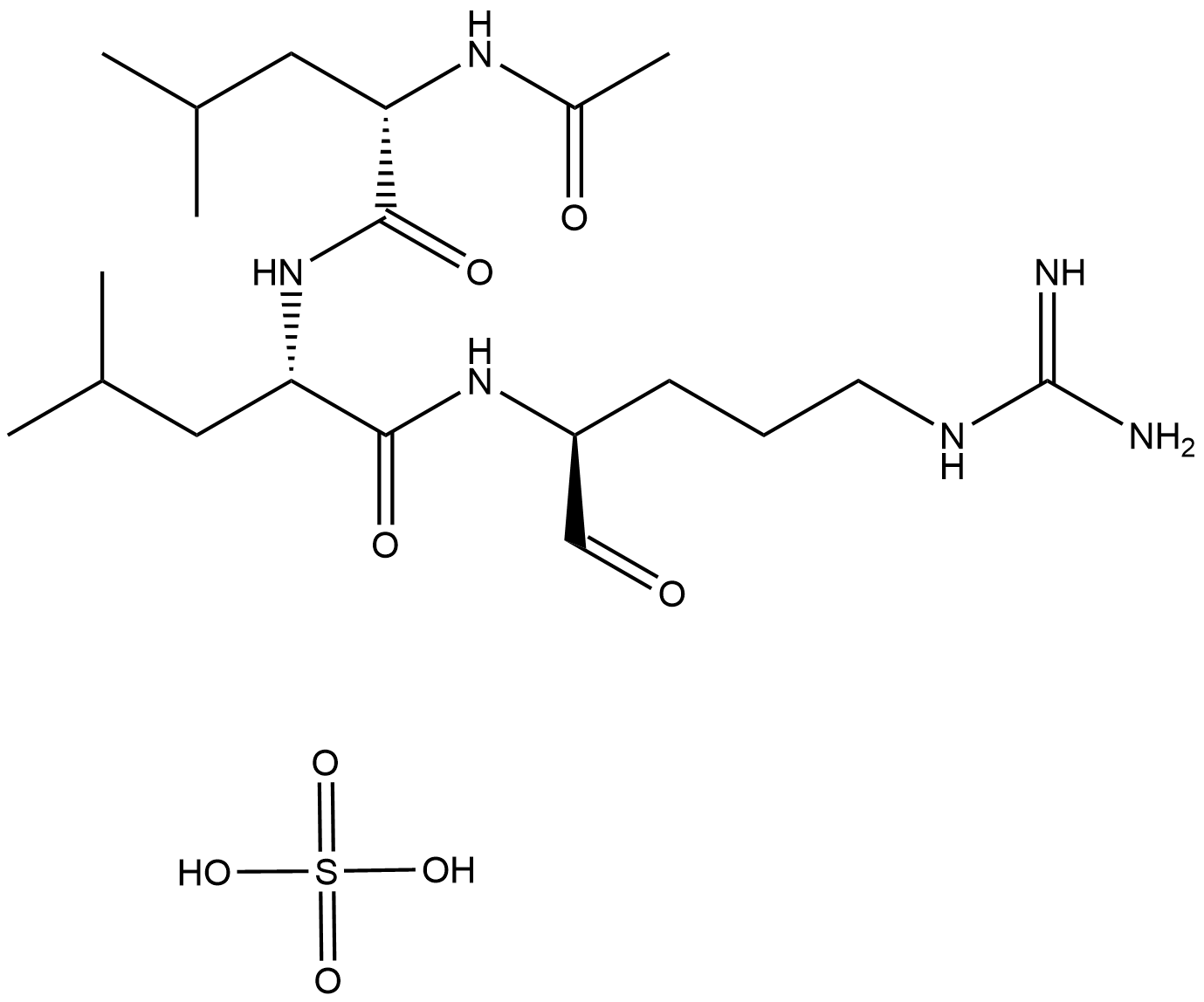 A2570 Leupeptin, MicrobialTarget: Cathepsins|Calpains|TrypsinsSummary: Inhibitor of serine and cysteine proteases
A2570 Leupeptin, MicrobialTarget: Cathepsins|Calpains|TrypsinsSummary: Inhibitor of serine and cysteine proteases -
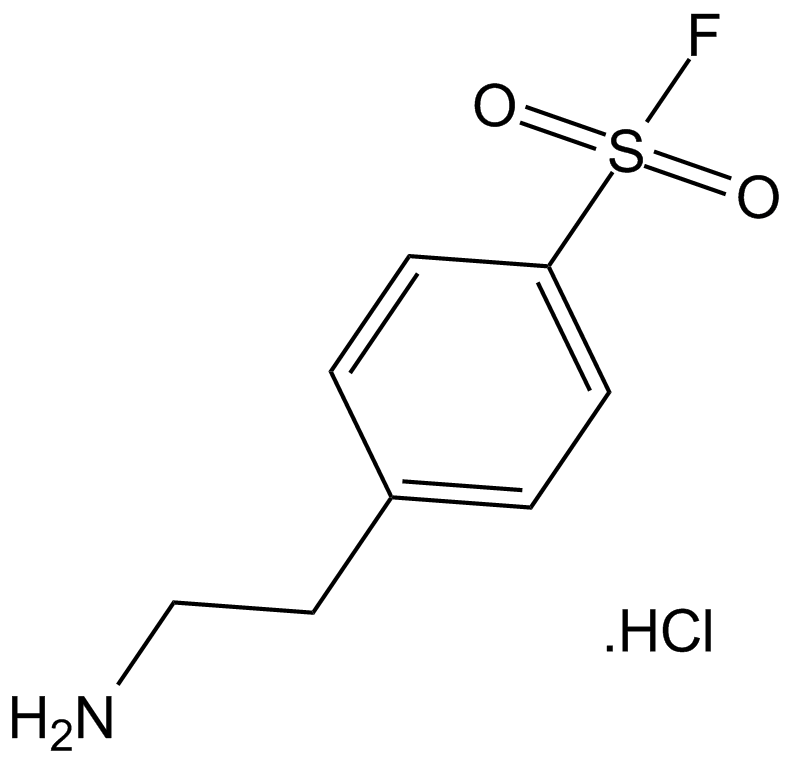 A2573 AEBSF.HCl1 CitationTarget: Serine ProteasesSummary: Serine protease inhibitor
A2573 AEBSF.HCl1 CitationTarget: Serine ProteasesSummary: Serine protease inhibitor -
 A2574 Aprotinin1 CitationTarget: Trypsin|Chymotrypsin|Kallikrein|TrypsinogenSummary: Inhibitor of bovine pancreatic trypsin
A2574 Aprotinin1 CitationTarget: Trypsin|Chymotrypsin|Kallikrein|TrypsinogenSummary: Inhibitor of bovine pancreatic trypsin -
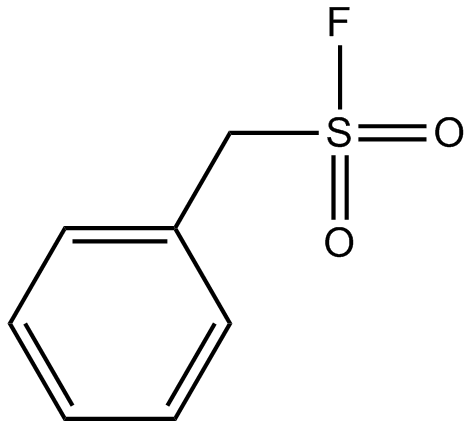 A2587 PMSF1 CitationTarget: Serine ProteasesSummary: Serine proteinases inhibitor, irreversible
A2587 PMSF1 CitationTarget: Serine ProteasesSummary: Serine proteinases inhibitor, irreversible

