Search results for: 'signaling pathways metabolism ido'
-
 A3483 IDO inhibitor 1Summary: Indoleamine-2,3-dioxygenase inhibitor
A3483 IDO inhibitor 1Summary: Indoleamine-2,3-dioxygenase inhibitor -
 L1044 DiscoveryProbe™ NF-κB Signaling LibrarySummary: A unique collection of 73 NF-κB inhibitors for NF-κB signaling pathway research.
L1044 DiscoveryProbe™ NF-κB Signaling LibrarySummary: A unique collection of 73 NF-κB inhibitors for NF-κB signaling pathway research. -
 L1026 DiscoveryProbe™ Neuronal Signaling Library1 CitationSummary: A unique collection of 556 neuronal signaling-related small molecules for neuroscience reasearch.
L1026 DiscoveryProbe™ Neuronal Signaling Library1 CitationSummary: A unique collection of 556 neuronal signaling-related small molecules for neuroscience reasearch. -
 A4300 GW96627 CitationSummary: PPARγ antagonist
A4300 GW96627 CitationSummary: PPARγ antagonist -
 A4381 FK866 (APO866)10 CitationTarget: NamptSummary: NAMPT inhibitor, non-competitive, highly specific
A4381 FK866 (APO866)10 CitationTarget: NamptSummary: NAMPT inhibitor, non-competitive, highly specific -
 L1044P DiscoveryProbe™ NF-κB Signaling Compound Library PlusSummary: A unique collection of 178 NF-κB inhibitors for NF-κB signaling pathway research.
L1044P DiscoveryProbe™ NF-κB Signaling Compound Library PlusSummary: A unique collection of 178 NF-κB inhibitors for NF-κB signaling pathway research. -
 L1026P DiscoveryProbe™ Neuronal Signaling Compound Library PlusSummary: A unique collection of 948 neuronal signaling-related small molecules for neuroscience reasearch.
L1026P DiscoveryProbe™ Neuronal Signaling Compound Library PlusSummary: A unique collection of 948 neuronal signaling-related small molecules for neuroscience reasearch. -
 A4304 Rosiglitazone5 CitationSummary: Potent PPARγ agonist
A4304 Rosiglitazone5 CitationSummary: Potent PPARγ agonist -
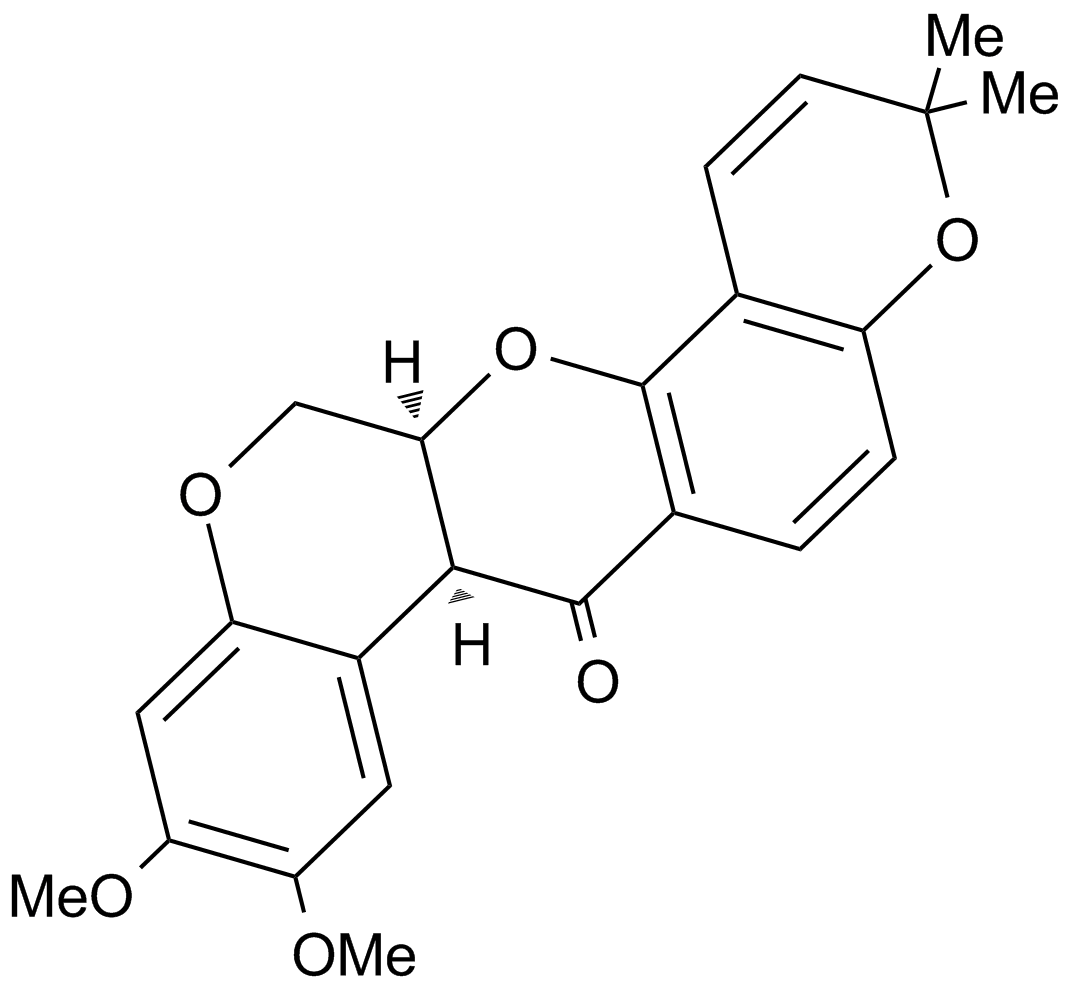 A4451 DeguelinSummary: Anticancer and antiviral agent
A4451 DeguelinSummary: Anticancer and antiviral agent -
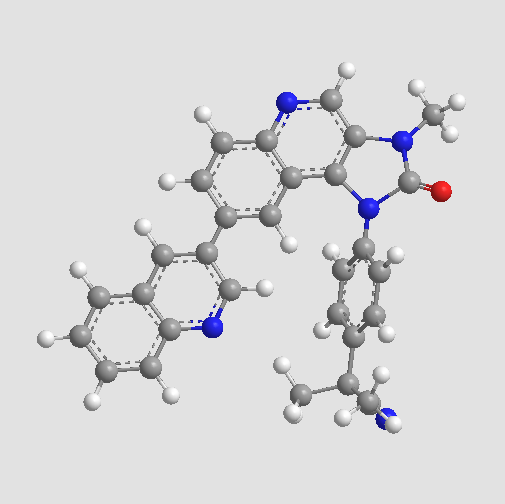 A8246 BEZ235 (NVP-BEZ235)2 CitationSummary: PI3K/mTOR inhibitor,ATP-competitve
A8246 BEZ235 (NVP-BEZ235)2 CitationSummary: PI3K/mTOR inhibitor,ATP-competitve


