INCB-024360 Epacadostat
Epacadostat (CAS 1204669-58-8) is an orally bioavailable small molecule inhibitor, functioning as a potent and selective indoleamine-2,3-dioxygenase 1 (IDO1) inhibitor in cellular systems and exhibiting strong antagonist activity against IDO1 in various tumor cell types. Additionally, it disrupts tryptophan metabolism and modulates immune cell responses by suppressing kynurenine production.
In preclinical experimental models, Epacadostat reduces IDO1 enzymatic activity with an IC50 value of 71.8 nM, tested against recombinant human IDO1 enzyme as well as in IFN-γ-stimulated cancer cell lines. It can also restore proliferation and cytokine production of T lymphocytes by preventing the accumulation of immunosuppressive metabolites within the tumor microenvironment.
In immuno-oncology research and drug development, Epacadostat is widely used for investigating the role of IDO1-mediated immune evasion in cancer, evaluating novel combinations with PD-1/PD-L1 checkpoint inhibitors, and assessing immune restoration in in vitro and in vivo studies. This compound serves as a valuable tool both for elucidating the mechanisms of tumor immune tolerance and for preclinical screening of potential therapeutic strategies targeting the tumor-immune interface.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 438.23 |
| Cas No. | 1204669-58-8 |
| Formula | C11H13BrFN7O4S |
| Synonyms | INCB024360 |
| Solubility | insoluble in H2O; ≥17.1 mg/mL in DMSO; ≥2.96 mg/mL in EtOH with ultrasonic |
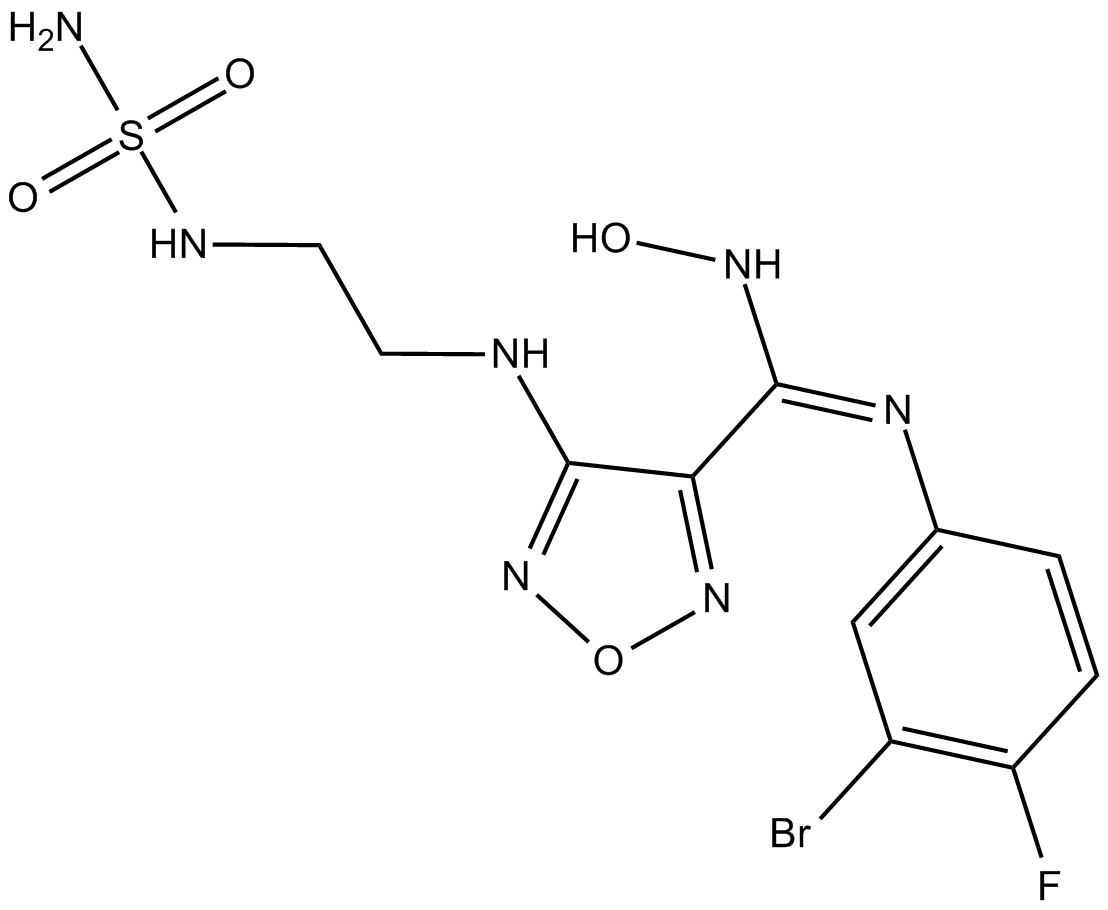
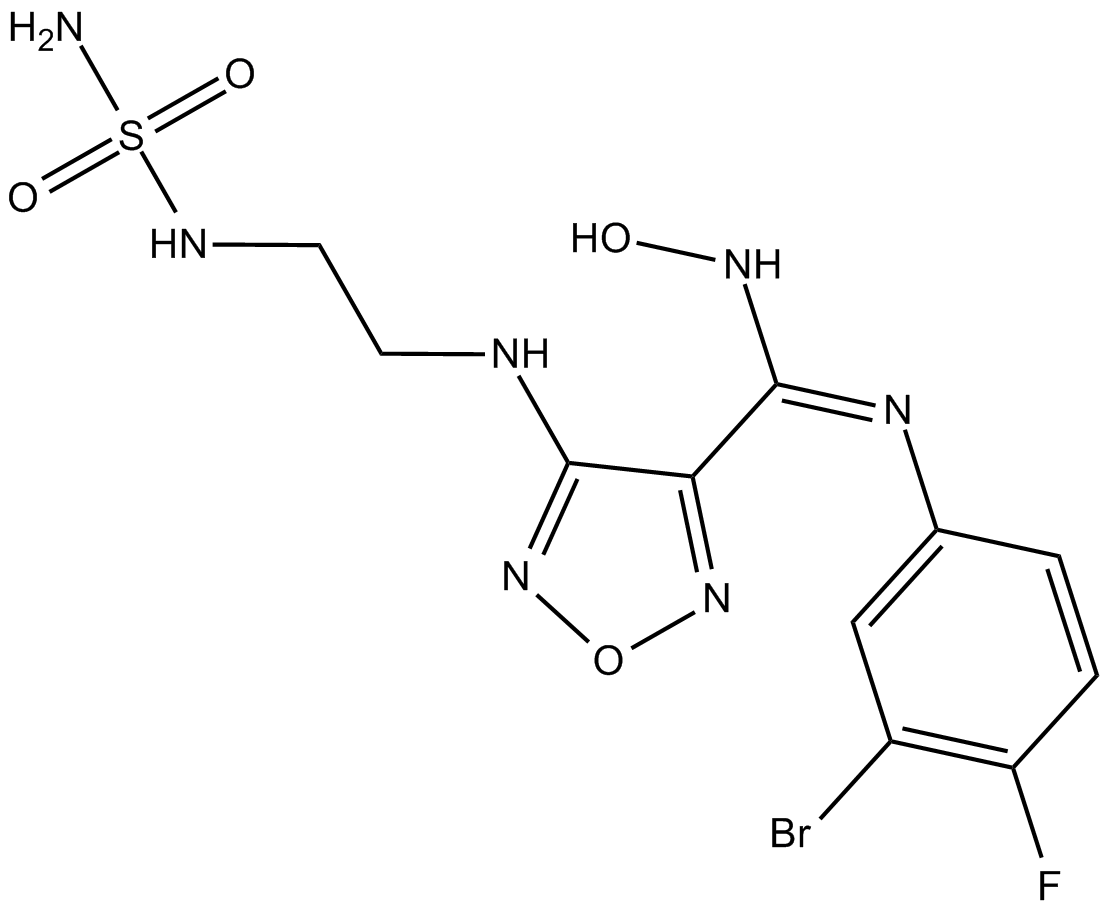
| Chemical Name | (E)-N'-(3-bromo-4-fluorophenyl)-N-hydroxy-4-((2-(sulfamoylamino)ethyl)amino)-1,2,5-oxadiazole-3-carboximidamide |
| SDF | Download SDF |
| Canonical SMILES | NS(NCCNc1n[o]nc1/C(\NO)=N\c(cc1)cc(Br)c1F)(=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Kinase assays |
The assays were performed at room temperature using 20 nM IDO and 2 mM D-Trp in the presence of 20 mM ascorbate, 3.5 μM methylene blue and 0.2 mg/mL catalase in 50 mM potassium phosphate buffer (pH 6.5). The initial reaction rates were recorded by continuously following the absorbance increase at 321 nm due to the formation of N’-formlylkynurenine. |
| Cell experiment [2]: | |
|
Cell lines |
HeLa cells and human T cells |
|
Preparation method |
The solubility of this compound in DMSO is >15.7mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0.001~2 μM |
|
Applications |
IDO1 induction significantly suppressed T-cell proliferation in coculture systems, and the suppression was effectively reversed by INCB024360. IDO1 also increases IFN-γ production, and reduces conversion to regulatory T (Treg)–like cells. |
| Animal experiment [2]: | |
|
Animal models |
C57BL/6 mice bearing IDO1-expressing PAN02 pancreatic carcinomas |
|
Dosage form |
25 and 100 mg/kg, orally, twice a day for 25 days |
|
Application |
The growth of tumors in syngeneic immunocompetent C57BL/6 mice was inhibited in a dose-dependent fashion. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Yue EW1, Douty B, Wayland B, et al. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. Med Chem. 2009 Dec 10;52(23):7364-7. [2] Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M et al: Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010 Apr 29;115(17):3520-30. |
|
| Description | INCB024360 is a potent inhibitor of IDO1 with an IC50 value of 10 nM. | |||||
| Targets | IDO1 | |||||
| IC50 | 10 nM | |||||
Quality Control & MSDS
- View current batch:
Chemical structure