Vorinostat (SAHA, MK0683)
Vorinostat (SAHA, suberoylanilide hydroxamic acid, CAS 149647-78-9) is a small-molecule histone deacetylase (HDAC) inhibitor, commonly utilized as a tool compound for epigenetic studies and cancer biology research. By inhibiting HDAC activity, vorinostat promotes histone acetylation, thereby inducing alterations in chromatin structure and gene expression. Mechanistically, it triggers apoptosis through activation of intrinsic apoptotic pathways, specifically influencing Bcl-2 family protein expression and prompting mitochondrial release of cytochrome C. Vorinostat has demonstrated activities in vitro and in vivo across diverse cancer cell lines, making it broadly applicable in experimental oncology, molecular signaling research, and investigations into epigenetic modulation. Reported IC50 values against HDAC range between 10–50 nM, depending on assay conditions and cell models employed.
Reference
[1] Hui-ming Z, Qian-hai D, Wei-ping C, Ru-bin L. Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF-kB nuclear translocation. International Immunopharmacology. 2013, 17. 329-335.
[2] Norihisa U, Sayaka K, Hisanori M, Katsuhiko Y, Airo T. Requirement of p38 MAPK for a cell-death pathway triggered by vorinostat in MDA-MB-231 human breast cancer cells. Cancer Letters. 2012, 315. 112-121.
- 1. Gabrielle L. Brumfield, Kenadie R. Doty, et al. "M344 Suppresses Histone Deacetylase-Associated Phenotypes and Tumor Growth in Neuroblastoma." Int J Mol Sci. 2025 Sep 1;26(17):8494. PMID: 40943415
- 2. Nicholas W Harper, Gavin A Birdsall, et al. "RNA Pol II inhibition activates cell death independently from the loss of transcription." Cell. 2025 Aug 14:S0092-8674(25)00856-6. PMID: 40818455
- 3. Nicholas W. Harper, Gavin A. Birdsall et al. "Pol II degradation activates cell death independently from the loss of transcription." bioRxiv. 2025 Jan 14:2024.12.09.627542. PMID: 39713309
- 4. Joseph Boyle, Derick Chiappo, et al. "Aminocoumarin-based heme oxygenase activity fluorescence probe reveals novel aspects of HO-1 regulation." Research Square. 22 Nov, 2023
- 5. Aran Merati, Spandana Kotian, et al. "Glioma Stem Cells Are Sensitized to BCL-2 Family Inhibition by Compromising Histone Deacetylases." Int J Mol Sci. 2023 Sep 5;24(18):13688. PMID: 37761989
- 6. Spandana Kotian, Rachel M Carnes, et al. "Enhancing Transcriptional Reprogramming of Mesenchymal Glioblastoma with Grainyhead-like 2 and HDAC Inhibitors Leads to Apoptosis and Cell-Cycle Dysregulation." Genes (Basel). 2023 Sep 12;14(9):1787. PMID: 37761927
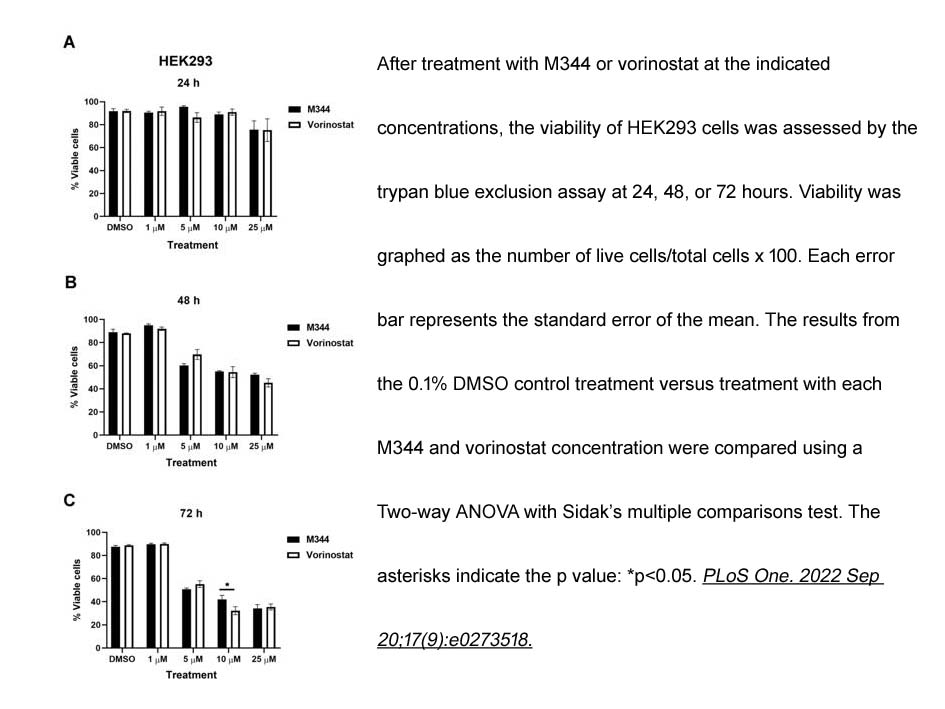
- 7. Shelby M. Knoche, Gabrielle L. Brumfield, et al. "The histone deacetylase inhibitor M344 as a multifaceted therapy for pancreatic cancer." PLoS One. 2022 Sep 20;17(9):e0273518. PMID: 36126055
- 8. Phoebe McCrorie, Jonathan Rowlinson, et al. "Detection of Label-Free Drugs within Brain Tissue Using Orbitrap Secondary Ion Mass Spectrometry as a Complement to Neuro-Oncological Drug Delivery." Pharmaceutics. 2022 Mar 5;14(3):571. PMID: 35335947
- 9. Nora-Guadalupe P Ramirez, Jeon Lee, et al. "ADAP1 promotes latent HIV-1 reactivation by selectively tuning KRAS–ERK–AP-1 T cell signaling-transcriptional axis." Nat Commun. 2022 Mar 1;13(1):1109. PMID: 35232997
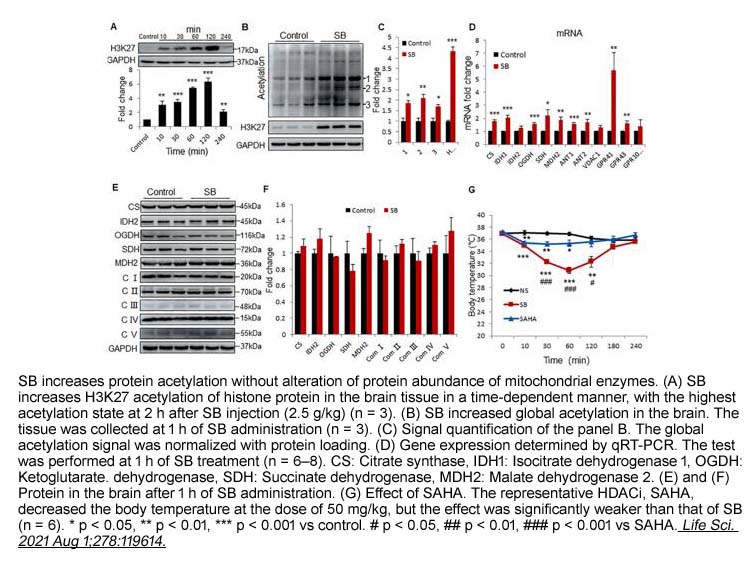
- 10. Yanhong Xu, Shiqiao Peng, et al. "High doses of butyrate induce a reversible body temperature drop through transient proton leak in mitochondria of brain neurons." Life Sci. 2021 Aug 1;278:119614 PMID: 34022200
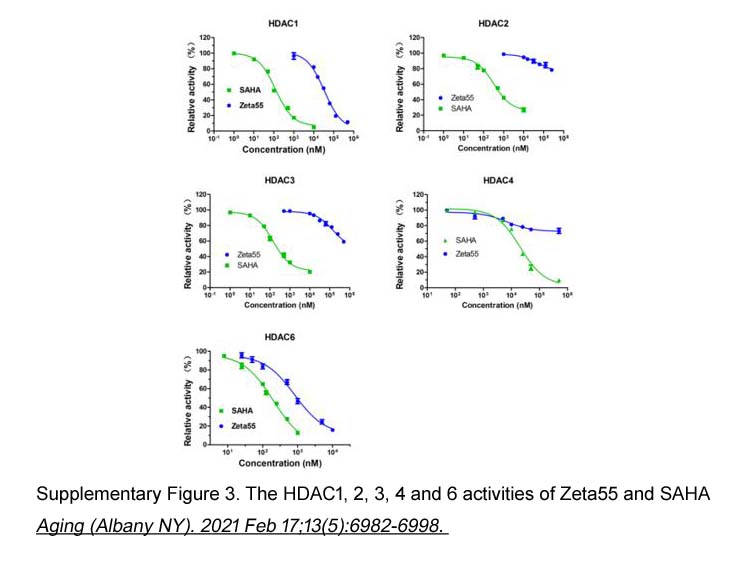
- 11. Maojun Zhou, Hao Zheng, et al. "Discovery of a novel AR/HDAC6 dual inhibitor for prostate cancer treatment." Aging (Albany NY). 2021 Feb 17;13(5):6982-6998 PMID: 33621955
- 12. Haiyang Yu, Shan Lu, et al. "TDP-43 and HSP70 phase separate into anisotropic, intranuclear liquid spherical annuli." bioRxiv. March 29, 2020
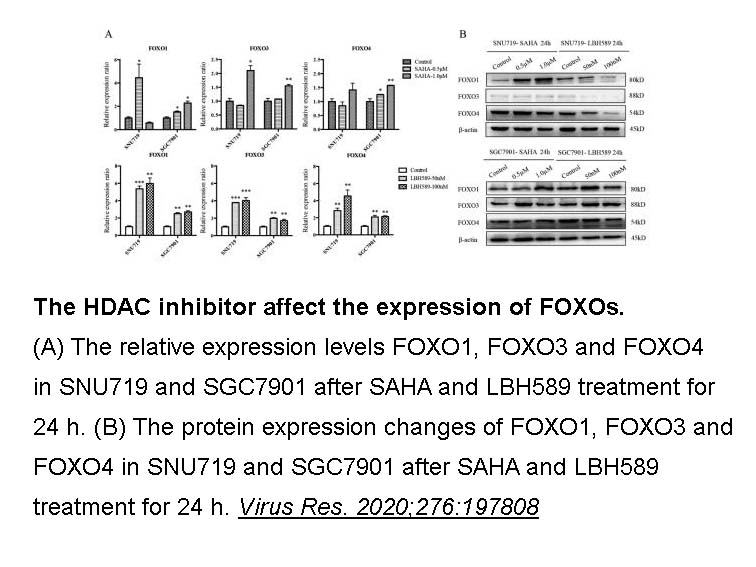
- 13. Liu W, Song YY, et al. "Dysregulation of FOXO transcription factors in Epstein-Barr virus-associated gastric carcinoma." Virus Res. 2020;276:197808 PMID: 31712122
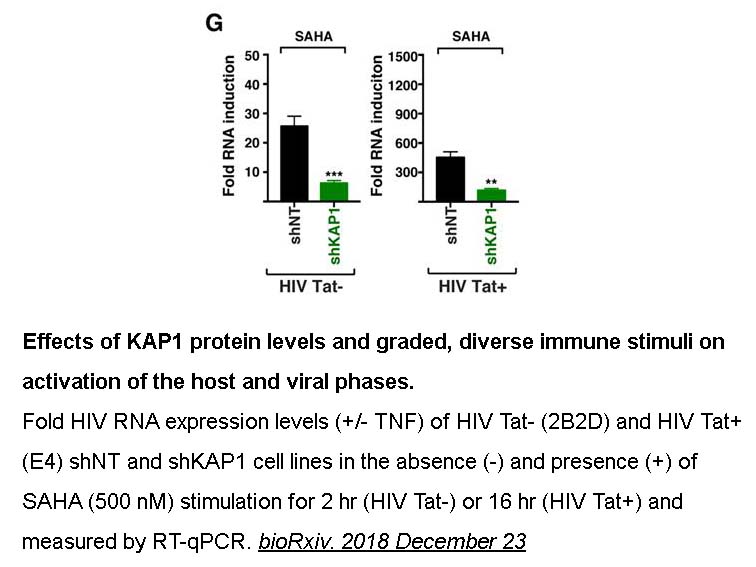
- 14. Emily L Morton, Christian V Forst, et al. "Transcriptional Circuit Fragility Influences HIV Proviral Fate." bioRxiv. 2018 December 23
- 15. Feng XL, Deng HB, et al. "Suberoylanilide Hydroxamic Acid Triggers Autophagy by Influencing the mTOR Pathway in the Spinal Dorsal Horn in a Rat Neuropathic Pain Model." Neurochem Res. 2018 Dec 17 PMID: 30560396
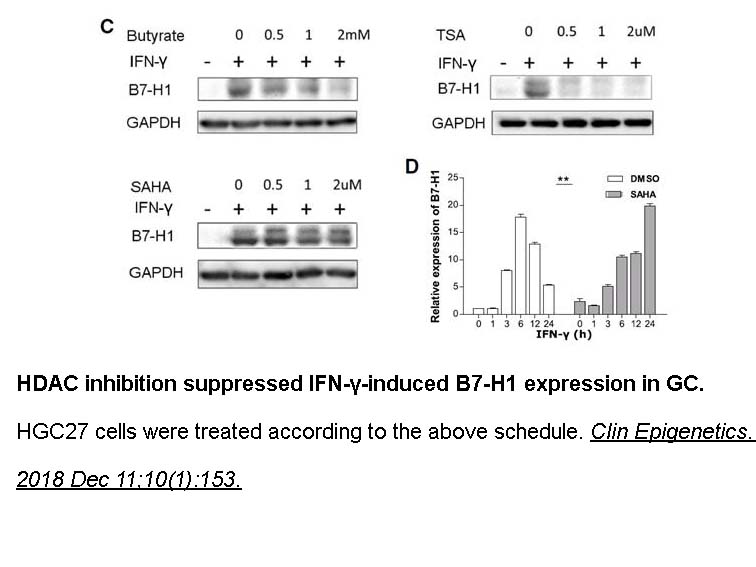
- 16. Deng R, Zhang P, et al. "HDAC is indispensable for IFN-γ-induced B7-H1 expression in gastric cancer." Clin Epigenetics. 2018 Dec 11;10(1):153 PMID: 30537988
- 17. Kim SR, Lewis JM, et al. "BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition." Oncotarget.2018 Jun 26;9(49):29193-29207 PMID: 30018745
- 18. Tuzlak S, Haschka MD, et al. "Differential effects of Vav-promoter-driven overexpression of BCLX and BFL1 on lymphocyte survival and B cell lymphomagenesis." FEBS J. 2018 Apr;285(8):1403-1418 PMID: 29498802
- 19. Mirza AN, Fry MA, et al. "Combined inhibition of atypical PKC and histone deacetylase 1 is cooperative in basal cell carcinoma treatment." JCI Insight. 2017 Nov 2;2(21). pii: 97071 PMID: 29093271
- 20. Hai Y, Shinsky SA, et al. "Histone deacetylase 10 structure and molecular function as a polyamine deacetylase." Nat Commun. 2017 May 18;8:15368 PMID: 28516954
- 21. Bagnall NH, Hines BM, et al. "Insecticidal activities of histone deacetylase inhibitors against a dipteran parasite of sheep, Lucilia cuprina." Int J Parasitol Drugs Drug Resist. 2017 Apr;7(1):51-60 PMID: 28110187
- 22. Sun, Yuefeng, et al. "Fe65 Suppresses Breast Cancer Cell Migration and Invasion through Tip60 Mediated Cortactin Acetylation." Scientific reports 5 (2015) PMID: 26166158
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 264.3 |
| Cas No. | 149647-78-9 |
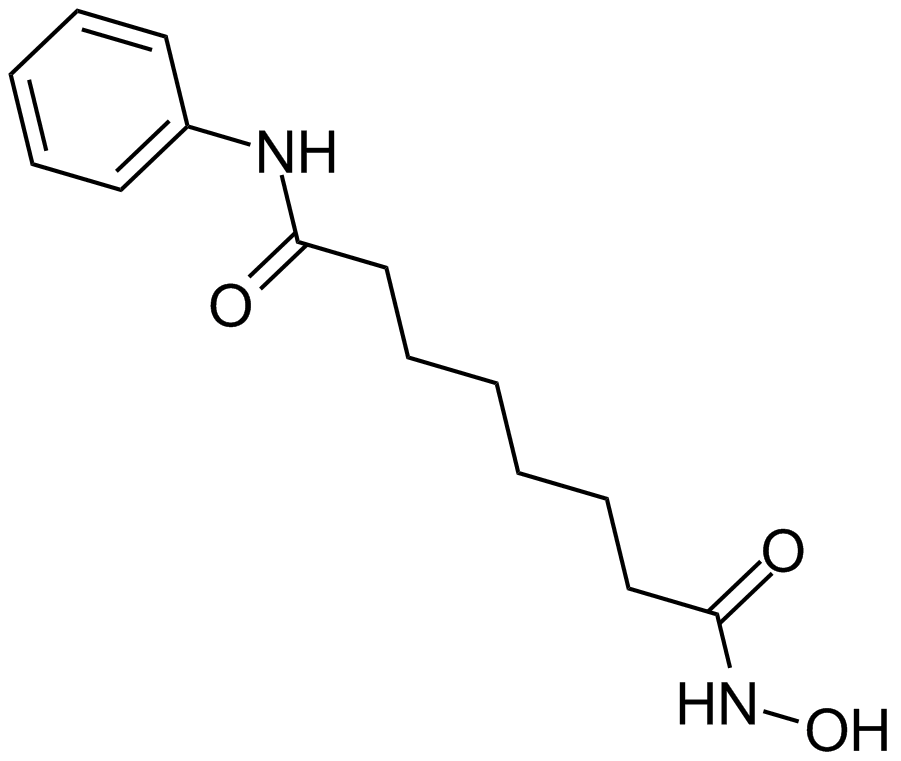
| Formula | C14H20N2O3 |
| Synonyms | SAHA, suberoylanilide hydroxamic acid, Suberanilohydroxamic acid, SAHA cpd |
| Solubility | insoluble in EtOH; insoluble in H2O; ≥4.41 mg/mL in DMSO |
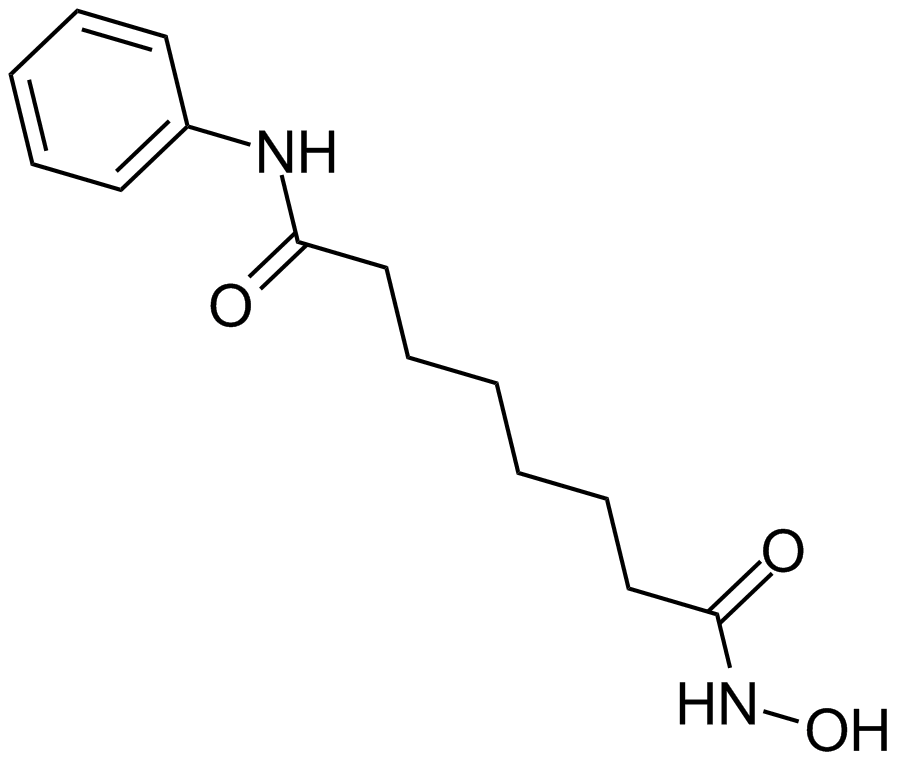
| Chemical Name | N'-hydroxy-N-phenyloctanediamide |
| SDF | Download SDF |
| Canonical SMILES | ONC(CCCCCCC(Nc1ccccc1)=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment: [1] | |
|
Cell lines |
Human cutaneous T-cell lymphomas (CTCL) cell lines |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
IC50: 0.146 μM (HH)、2.062 μM (HuT)、78 2.697 μM (MJ)、1.375 μM (MylA)、1.510 μM (SeAx); 72h |
|
Applications |
Vorinostat dose-dependently reduced cell proliferation with IC50 values of 0.146 μM, 2.062 μM, 2.697 μM, 1.375 μM and 1.510 μM in HH, HuT78, MJ, MylA and SeAx cells, respectively. |
| Animal experiment : [2] | |
|
Animal models |
C57BL/6 mice bearing Eμ-myc lymphomas |
|
Dosage form |
C57BL/6 mice bearing Eμ-myc lymphomas were injected with vorinostat (200 mg/kg i.p.) and lymphoma cells were harvested after the indicated time points. The percentage of tumor cells in the lymph node of C57BL/6 mice bearing Eμ-myc lymphomas treated with vorinostat was determined by FACS analysis. |
|
Applications |
Vorinostat induced a marked accumulation of Eμ-myc lymphomas displaying DNA fragmentation in vivo. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Wozniak M B, Villuendas R, Bischoff J R, et al. Vorinostat interferes with the signaling transduction pathway of T cell receptor and synergizes with PI3K inhibitors in cutaneous T-cell lymphoma. haematologica, 2010: haematol. 2009.013870. [2] Lindemann R K, Newbold A, Whitecross K F, et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proceedings of the National Academy of Sciences, 2007, 104(19): 8071-8076. |
|
| Description | Vorinostat (suberoylanilide hydroxamic acid, SAHA) is an inhibitor of HDAC with IC50 of ~10 nM. | |||||
| Targets | HDAC | |||||
| IC50 | ~10 nM | |||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data

Related Biological Data
Related Biological Data