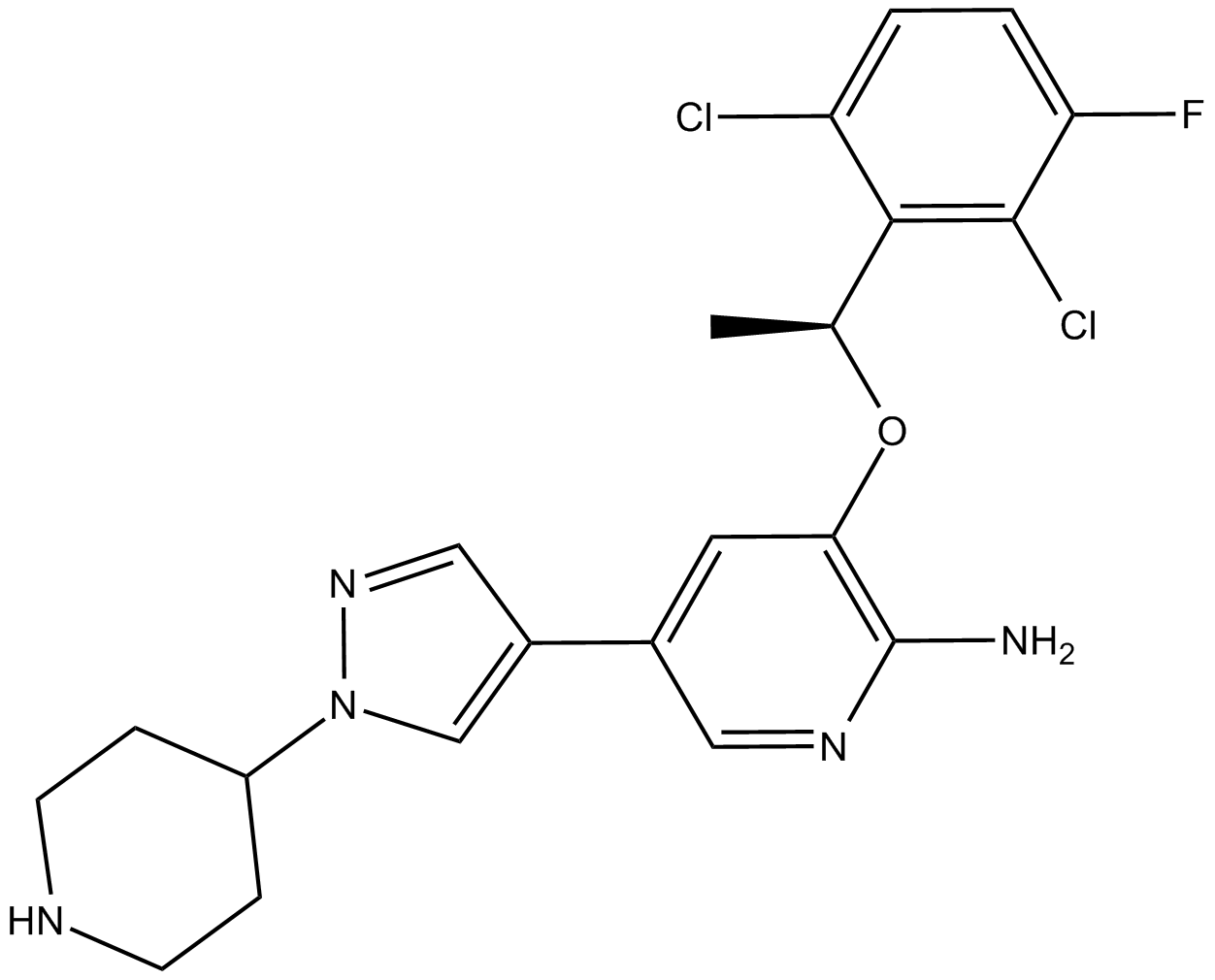
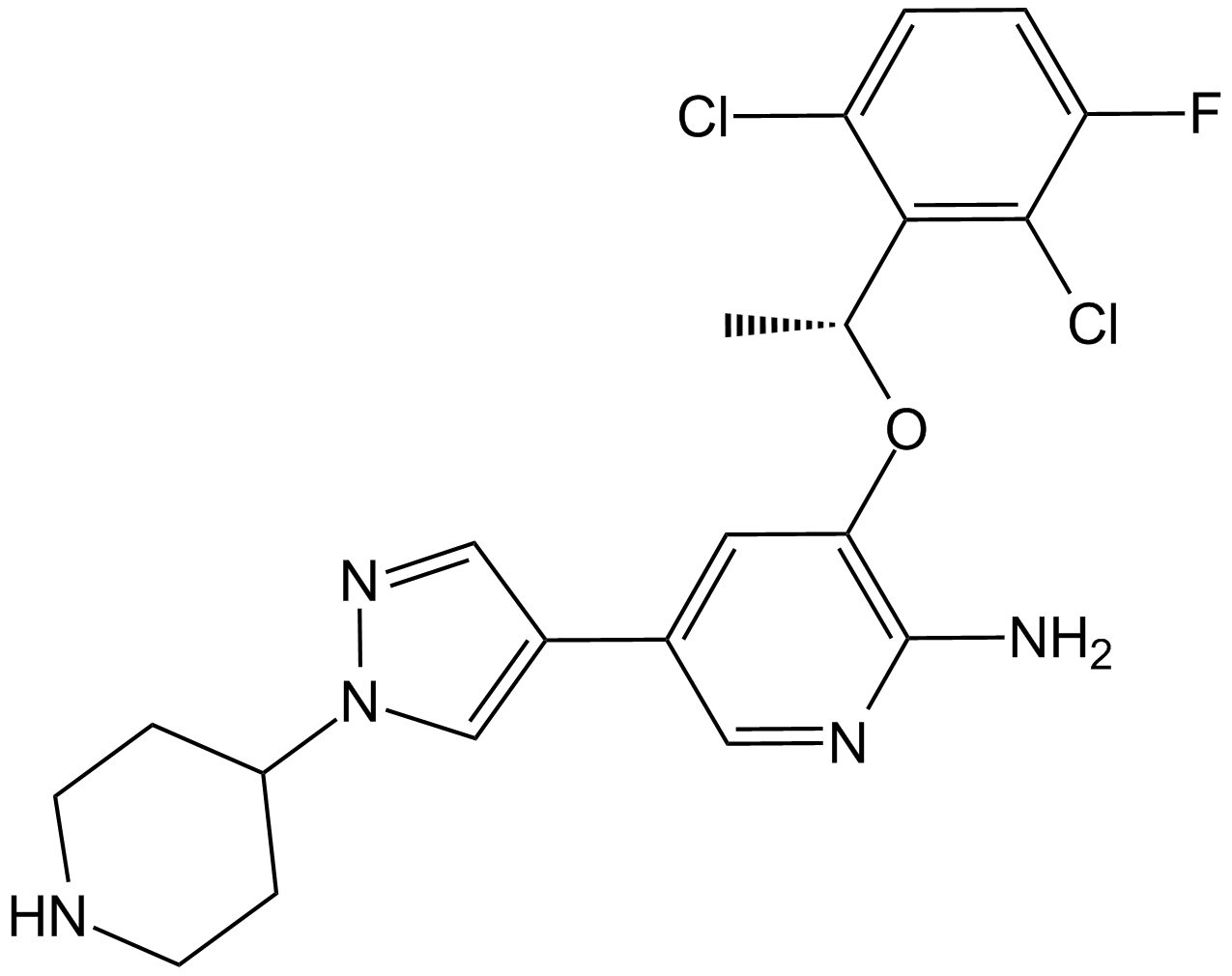
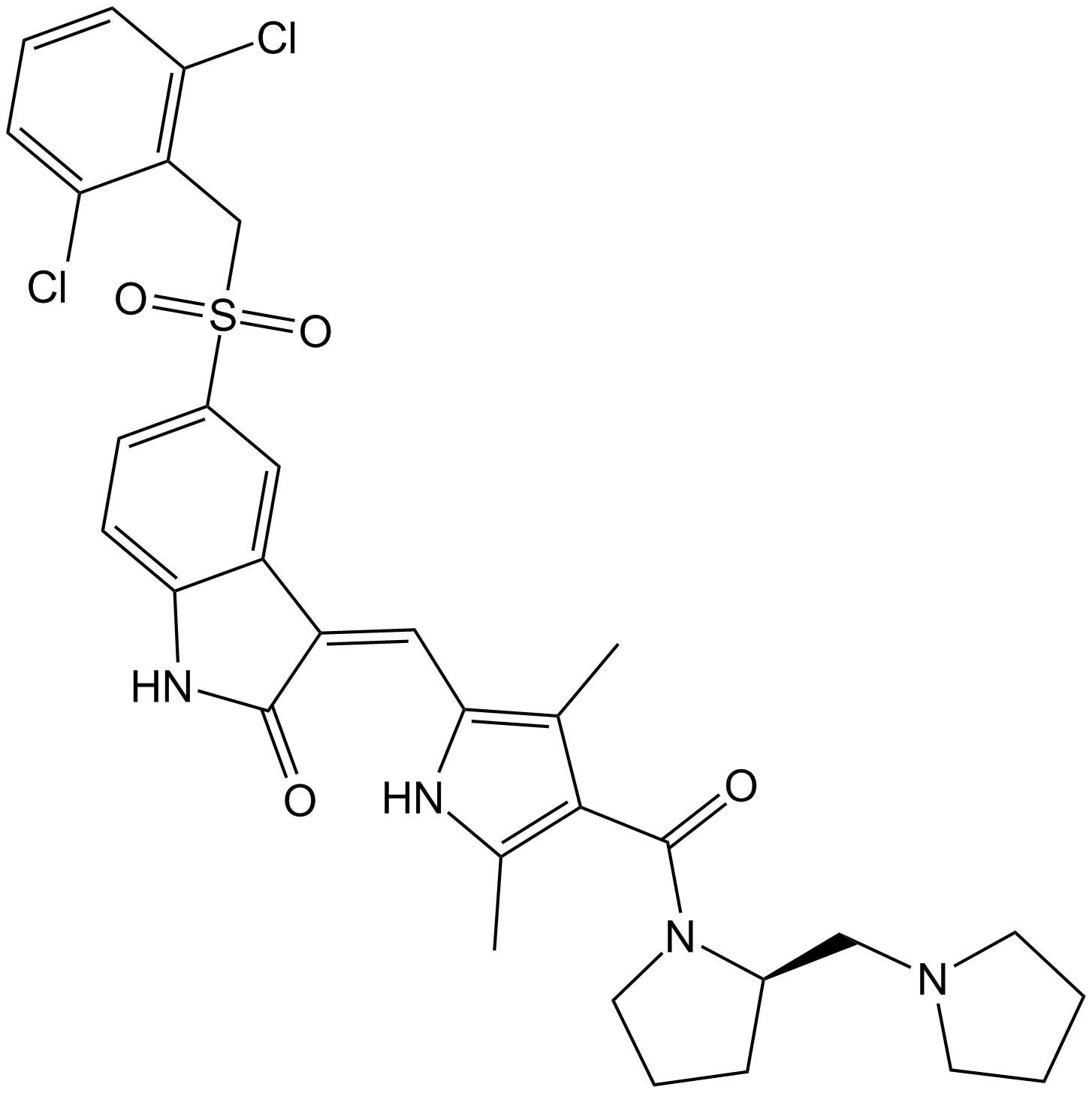
(S)-Crizotinib
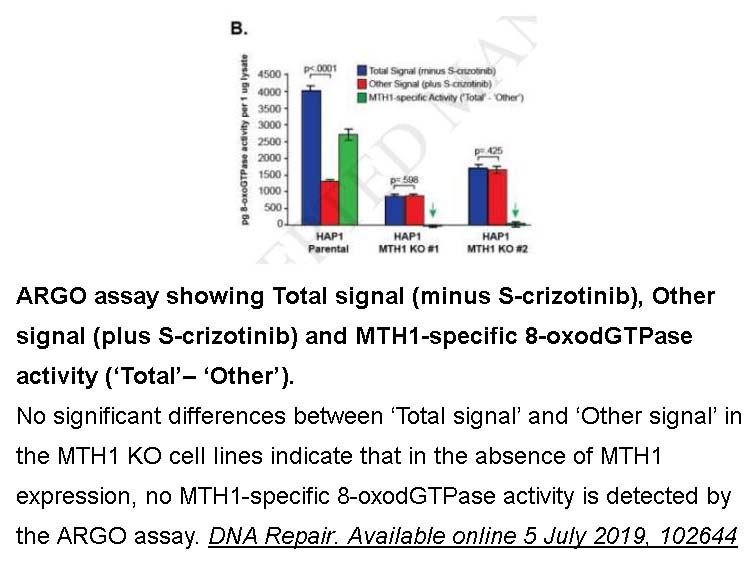
- 1. Lisa A.McPhersona, Clara I.Troccolibc, et al. "Increased MTH1-specific 8-oxodGTPase activity is a hallmark of cancer in colon, lung and pancreatic tissue." DNA Repair. Available online 5 July 2019, 102644.
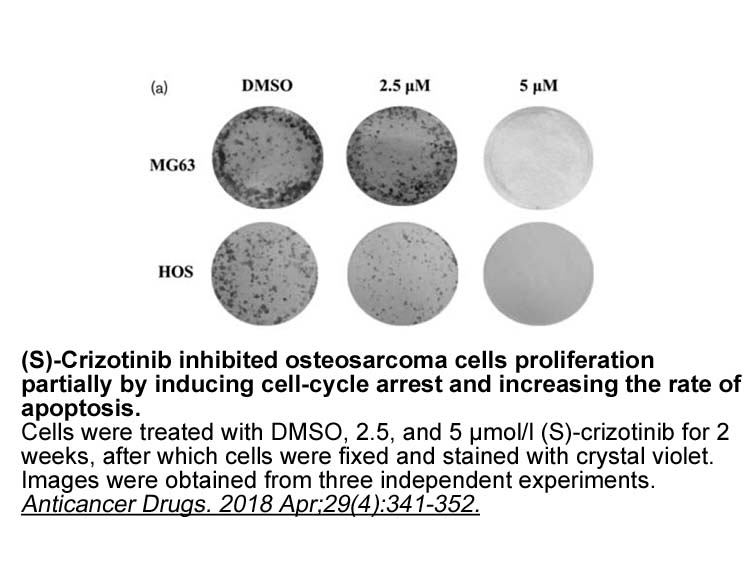
- 2. Qing X, Shao Z, et al. "Anticancer effect of (S)-crizotinib on osteosarcoma cells by targeting MTH1 and activating reactive oxygen species." Anticancer Drugs. 2018 Apr;29(4):341-352. PMID:29420337
- 3. Stewart E, Federico SM, et al. "Orthotopic patient-derived xenografts of paediatric solid tumours." Nature. 2017 Sep 7;549(7670):96-100. PMID:28854174
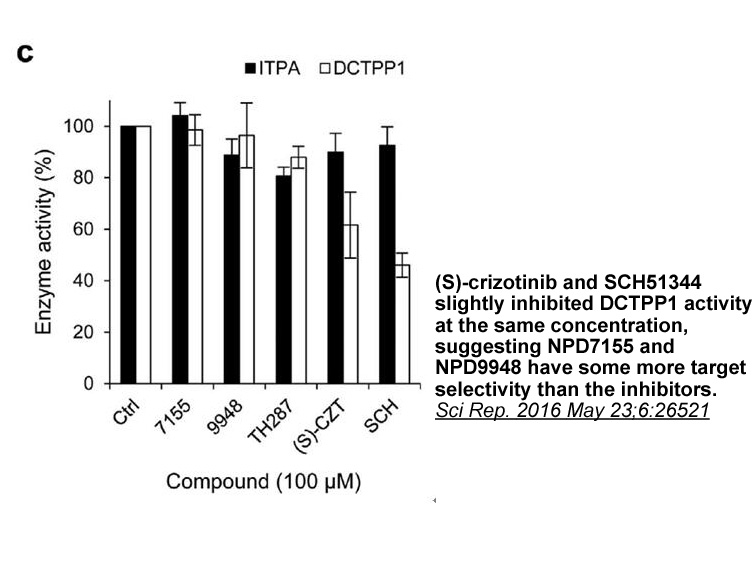
- 4. Kawamura T, Kawatani M, et al. "Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival." Sci Rep. 2016 May 23;6:26521. PMID:27210421
- 5. J Adachi, et al. "Proteome-wide discovery of unknown ATP-binding proteins and kinase inhibitor target proteins using an ATP probe." J Proteome Res. 2014 Sep 17. PMID:25230287
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 450.34 |
| Cas No. | 1374356-45-2 |
| Formula | C21H22Cl2FN5O |
| Solubility | ≥33.33 mg/mL in DMSO; insoluble in H2O; ≥8.58 mg/mL in EtOH with ultrasonic |
| Chemical Name | 3-[(1S)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-(1-piperidin-4-ylpyrazol-4-yl)pyridin-2-amine |
| SDF | Download SDF |
| Canonical SMILES | C[C@@H](c(c(Cl)c(cc1)F)c1Cl)Oc1c(N)ncc(-c2c[n](C3CCNCC3)nc2)c1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[1] | |
|
Cell lines |
BJ, H1437, H2122, H23, H358, H460, HCT116 and U2OS cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
BJ, SV40T, RASV12-cells (5 μM, 3h); U2OS cells (5 μM, 24h) |
|
Applications |
(S)-crizotinibthe selectively inhibited MTH1 catalytic activity with IC50 of 72 nM, while clinically used (R)-enantiomer of the drug was inactive with IC50 of 1375 nM. Furthermore, direct-binding assays (ITC) indicated a 16-fold higher affinity of the (S)-enantiomer towards MTH1 compared with (R)-enantiomer. By using Km concentrations of substrates, the average IC50 values for (S)-crizotinib and the MTH1 substrates 8-oxo-dGTP and 2-OH-dATP were 330 nM and 408 nM respectively. (S)-crizotinib efficiently inhibited colony formation of SW480 cells andKRAS-mutated PANC1 cells, similar to SCH51344. In addition, in vitro Kd measurements indicated that (S)-crizotinib was considerably less potent than the (R)-enantiomer against the established targets ALK,MET and ROS1. (S)-crizotinib did not lead to the detection of any significant effects on proliferation in SW480 cells and showed highest toxicity towards the SV40T and KRASV12 cells. (S)-crizotinib, in contrast to (R)-crizotinib, efficiently stabilized MTH1 validating the differential targeting within BJ-KRASV12 cells using a cellular thermal shift assay. (S)-crizotinib induced an increase in DNA single-strand breaks, activated DNA repair in human colon carcinoma cells, and effectively suppressed tumour growth in animal models as a result of disruption of nucleotide pool homeostasis via MTH1 inhibition. |
| Animal experiment:[2] | |
|
Animal models |
SCID mice (female, 5–6 weeks) |
|
Dosage form |
25 mg per kg,subcutaneously daily; 50 mg per kg, orally, daily |
|
Applications |
In vivo mouse xenograft studies showed (S)-crizotinib, but not the (R)-enantiomer, was able to impair overall tumour progression aswell as specifically reduce tumour volume by more than 50%. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: 1. Huber KVM, Salah E, Radic B, et al. Stereospecific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. NATURE,2014;508:222-227 |
|
| Description | (S)-crizotinib, the (S)-enantiomer of crizotinib, is a potent inhibitor of the human mutT homologue MTH1 (NUDT1) with an IC50 value of 72 nM. | |||||
| Targets | MTH1 | |||||
| IC50 | 72 nM | |||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data

Related Biological Data
Related Biological Data
Related Biological Data