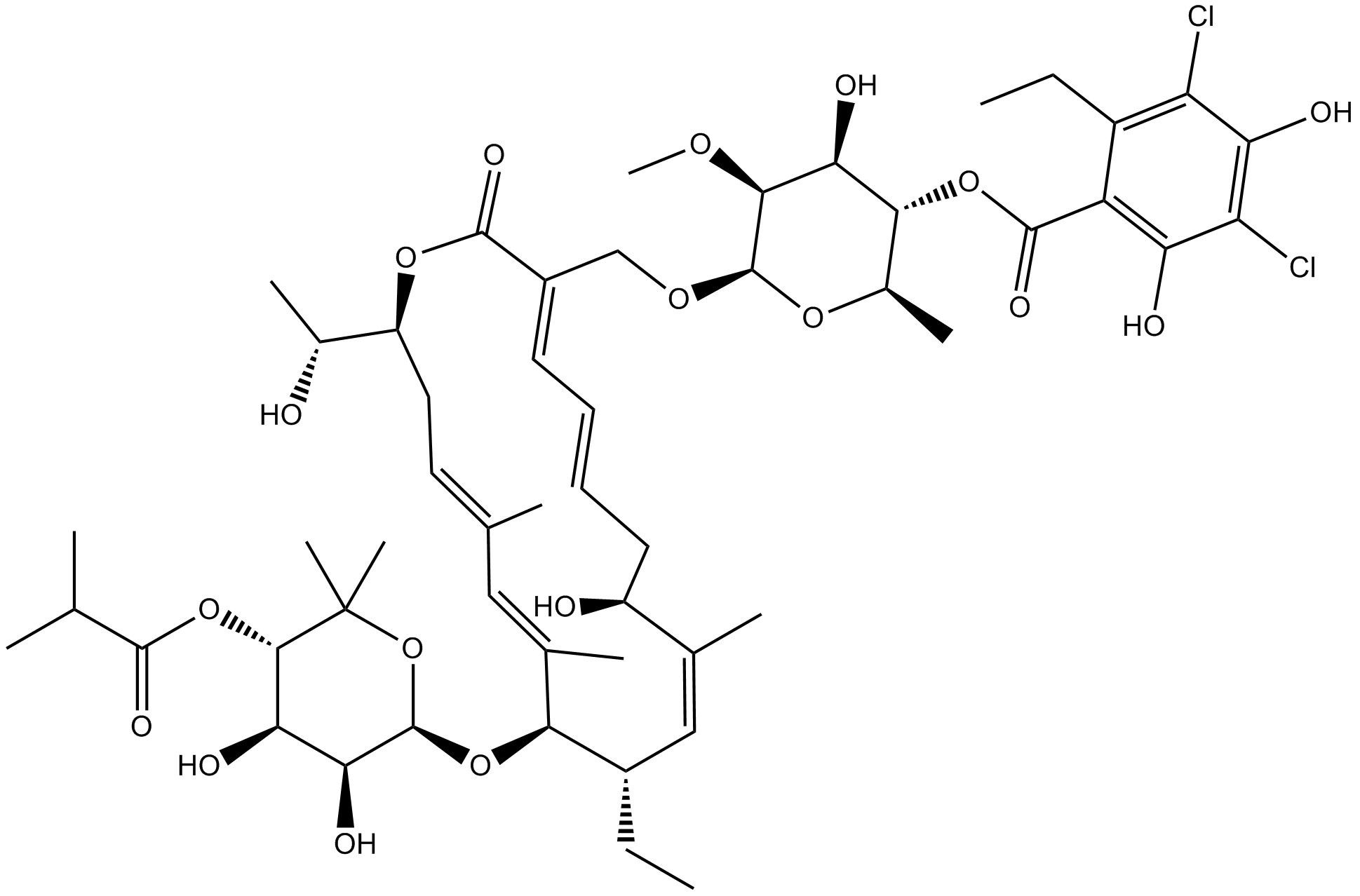
Fidaxomicin
Fidaxomicin is a macrocyclic antibiotic that inhibits RNA polymerase sigma subunit [1].
Antibiotics are a type of antimicrobial used in the treatment of bacterial infection. They can inhibit the growth of bacteria. Bacterial RNA polymerase mediates RNA synthesis.
Fidaxomicin is a narrow spectrum antibiotic that inhibits RNA polymerase sigma subunit. Fidaxomicin was a macrocyclic-lactone antibiotic produced by Actinomycete species. Fidaxomicin inhibited RNA polymerase in Clostridium difficile [1].
In patients infected with Clostridium difficile, fidaxomicin exhibited a higher clinical cure rate and a lower recurrence rate. In rats, fidaxomicin was safe by the intravenous route and exhibited LD50 value of 200 mg/kg. Fidaxomicin for the treament of Clostridium difficile infection (CDI) had entered Phase III trials [2]. In patients with CDI, fidaxomicin exhibited a lower reappearance of toxin in fecal filtrates and inhibited recurrence of CDI. Also, fidaxomicin preserved the intestinal microbiome during the treatment of CDI [3].
References:
[1]. Srivastava A, Talaue M, Liu S, et al. New target for inhibition of bacterial RNA polymerase: 'switch region'. Curr Opin Microbiol, 2011, 14(5): 532-543.
[2]. Poxton IR. Fidaxomicin: a new macrocyclic, RNA polymerase-inhibiting antibiotic for the treatment of Clostridium difficile infections. Future Microbiol, 2010, 5(4): 539-548.
[3]. Louie TJ, Cannon K, Byrne B, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis, 2012, 55 Suppl 2: S132-S142.
- 1. Giovanni Andrea Vitale, Shu-Ning Xia, et al. "Enhancing tandem mass spectrometry-based metabolite annotation with online chemical labeling." Nat Commun. 2025 Jul 26;16(1):6911 PMID: 40715063
- 2. Thomas A. Auchtung, Armando I. Lerma, et al. "Evaluating Effects of Antibiotics Across Preclinical Models of the Human Gastrointestinal Microbiota." Microbiologyopen. 2025 Aug;14(4):e70030. PMID: 40668040
- 3. Xiaoyun Huang, April E. Johnson, et al. "Clostridioides difficile colonization is not mediated by bile salts and utilizes Stickland fermentation of proline in an in vitro model." mSphere. 2025 Feb 25;10(2):e0104924. PMID: 39817755
- 4. Xiaoyun Huang, April E Johnson, et al. "Clostridioides difficile colonization is not mediated by bile salts and requires Stickland fermentation of proline in an in vitro model of infection" bioRxiv. 2024 Jul 17:2024.07.17.603937
- 5. April Elizabeth Johnson. "Utilization of Probiotics to Compete with Clostridioides difficile for Nutrient-Niches in a Variety of in vitro Contexts." University of Nebraska-Lincoln July 2024
- 6. Johnstone, Michael A. "Exploring the Physiology of Clostridioides difficile: Selenium-Dependent Catabolism of Host-Derived Nutrients."University of Central Florida. May 2024
- 7. Liang Meng Wee, Alexander B Tong, et al. "A trailing ribosome speeds up RNA polymerase at the expense of transcript fidelity via force and allostery." Cell. 2023 Mar 16;186(6):1244-1262.e34. PMID: 36931247
- 8. Michael A. Johnstone, Alexander D. Landgraf, et al. "Evaluation of Derivatives of (+)-Puupehenone againstClostridioides difficileand Other Gram-Positive Bacteria." ACS Omega. 2022 Sep 9;7(37):33511-33517. PMID: 36157757
- 9. Mody D, Athamneh AIM, et al. "Curcumin: A natural derivative with antibacterial activity against Clostridium difficile." J Glob Antimicrob Resist. 2019 Oct 14. pii: S2213-7165(19)30259-0. PMID: 31622683
- 10. AbdelKhalek A, Abutaleb NS, et al. "Antibacterial and antivirulence activities of auranofin against Clostridium difficile." Int J Antimicrob Agents. 2019 Jan;53(1):54-62. PMID: 30273668
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 1058.04 |
| Cas No. | 873857-62-6 |
| Formula | C52H74Cl2O18 |
| Solubility | ≥35.27 mg/mL in DMSO; insoluble in H2O; ≥12.25 mg/mL in EtOH |
| Chemical Name | (2R,3S,4S,5S,6R)-6-(((3E,5E,8S,9Z,11S,12R,13Z,15Z,18S)-12-(((2R,3S,4R,5S)-3,4-dihydroxy-5-(isobutyryloxy)-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-11-ethyl-8-hydroxy-18-((R)-1-hydroxyethyl)-9,13,15-trimethyl-2-oxooxacyclooctadeca-3,5,9,13,15-pentaen-3-yl |
| SDF | Download SDF |
| Canonical SMILES | C[C@@H](O)[C@@](C/C=C(C)\C=C(C)/[C@H](O[C@]1([H])OC(C)(C)[C@@H](OC(C(C)C)=O)[C@H](O)[C@@H]1O)[C@@H](CC)/C=C(C)\[C@@H](O)C/C=C/C=C2\CO[C@H]3[C@@H](OC)[C@@H](O)[C@H](OC(C4=C(C(Cl)=C(O)C(Cl)=C4O)CC)=O)[C@@H](C)O3)([H])OC2=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment: | |
|
Cell lines |
Human colonic fibroblasts |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
20 μM for 6 h; |
|
Applications |
Fidaxomicin (20 μM) prevented toxin A-induced cell rounding in human colonic CCD-18Co fibroblasts [1]. Moreover, Fidaxomicin suppressed toxin production in Clostridium difficile [2]. |
| Animal experiment: | |
|
Animal models |
Male C57BL/6 mice model |
|
Dosage form |
5 to 20 μM |
|
Applications |
Treatment with fidaxomicin (20 μM) significantly inhibited toxin A-mediated histologic damage and reduced the mean histology score and ileal IL-1β protein and mRNA expression in the mouse ileum [1]. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: 1. Koon, H. W., Ho, S., Hing, T. C., Cheng, M., Chen, X., Ichikawa, Y., Kelly, C. P. and Pothoulakis, C. (2014) Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum. Antimicrob Agents Chemother. 58, 4642-4650 2. Babakhani, F., Bouillaut, L., Sears, P., Sims, C., Gomez, A. and Sonenshein, A. L. (2013) Fidaxomicin inhibits toxin production in Clostridium difficile. J Antimicrob Chemother. 68, 515-522 |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data

Related Biological Data

Related Biological Data