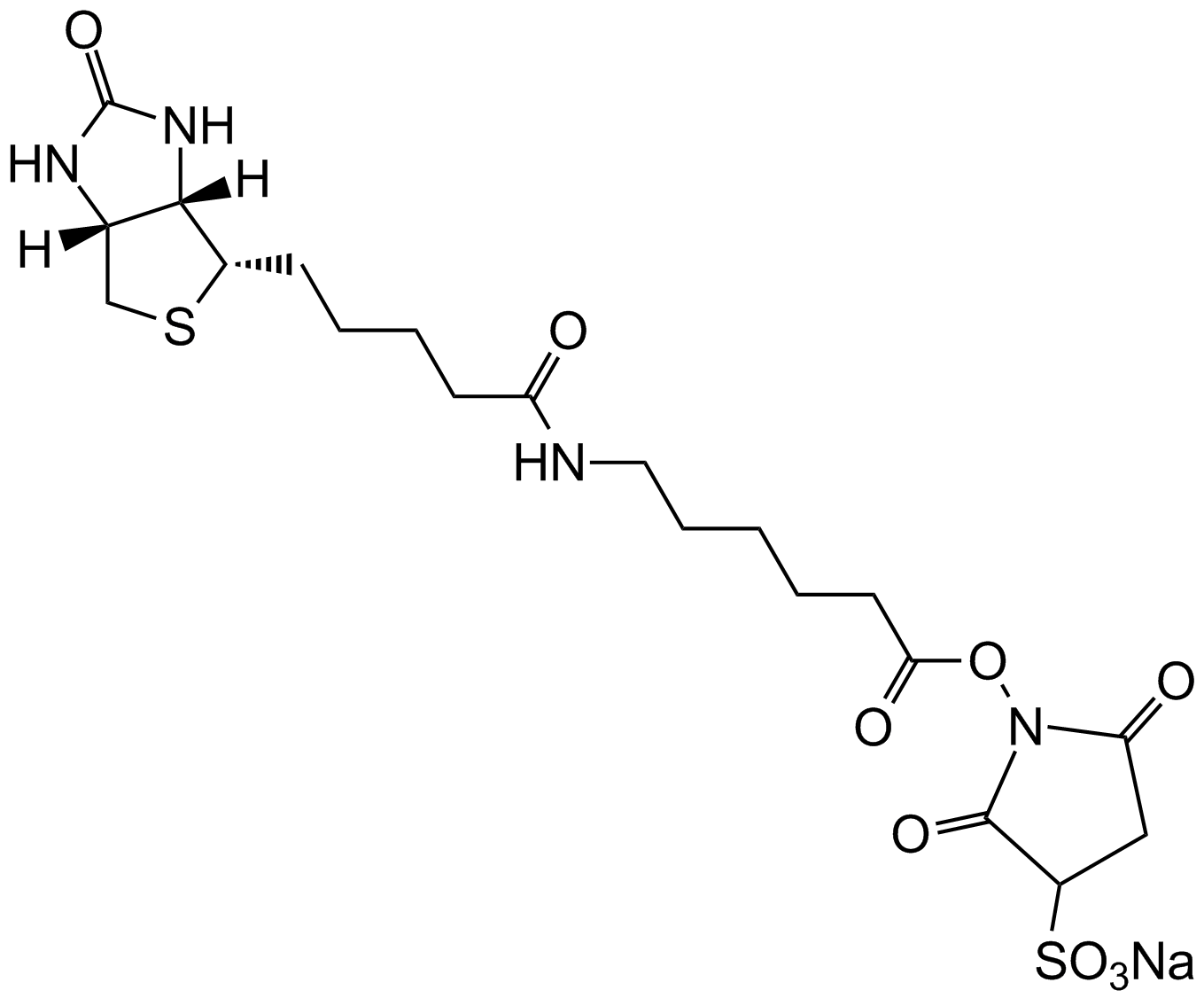
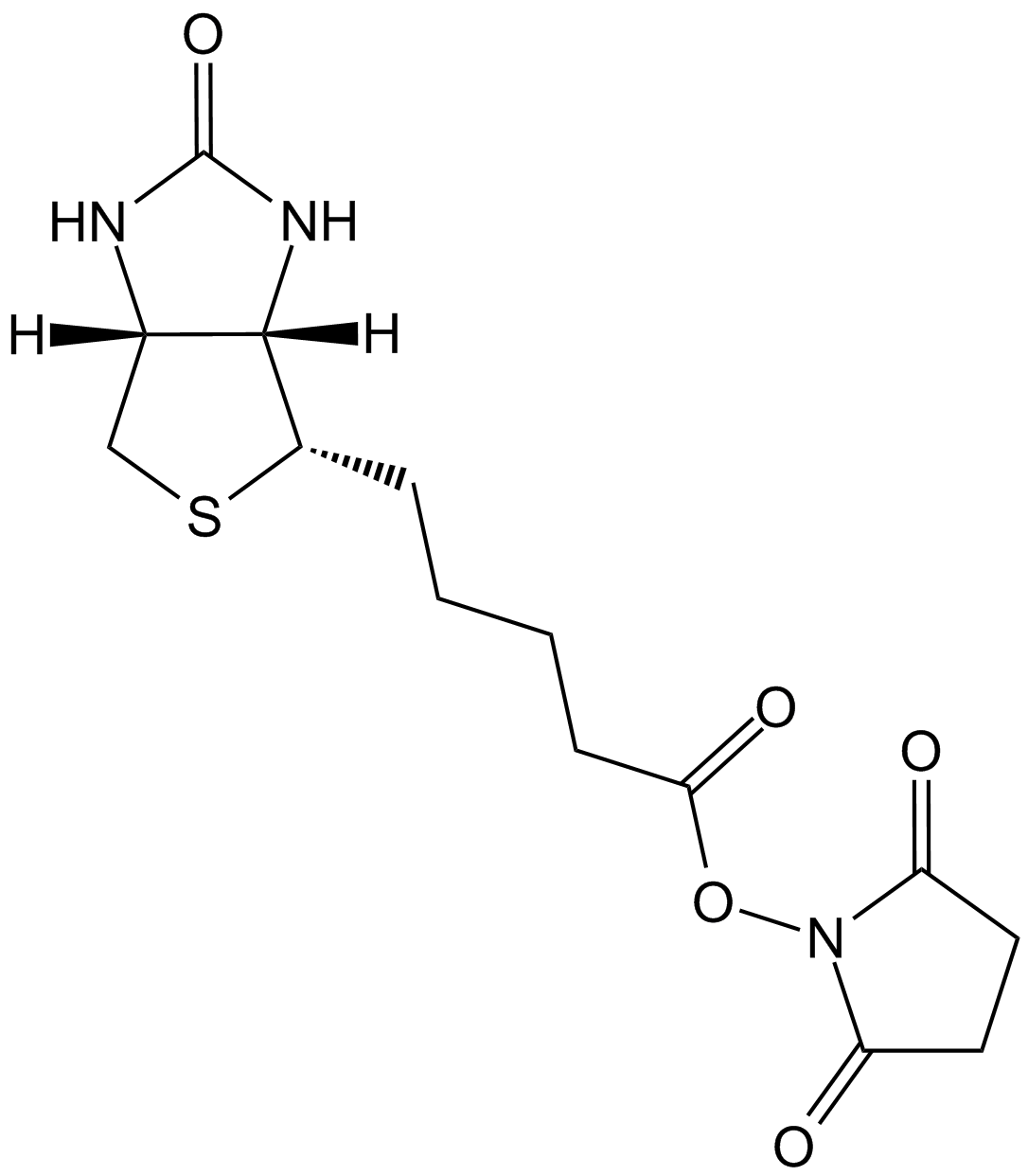
NHS-LC-Biotin
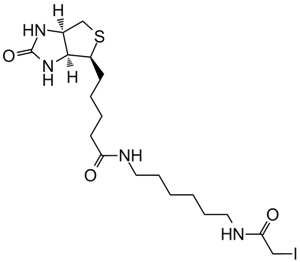
NHS-LC-Biotin, also known as NHS-X-biotin, is a biotinylation reagent designed to tag proteins or biomolecules through covalent conjugation with primary amines. Structurally, it consists of a biotin moiety connected via a hexanoic acid spacer arm terminated with an N-hydroxysuccinimide (NHS) ester group. The NHS ester reacts selectively with free amine groups on target proteins or other molecules, forming stable amide bonds to yield biotinylated conjugates accessible to subsequent detection and purification steps via streptavidin or avidin affinity. The hexanoic acid spacer enhances spatial separation between the biotin residue and the target molecule, improving binding accessibility in applications including immunoblotting, affinity chromatography, protein localization, and biomolecule labeling in biochemical research.
Reference
Bioconjugate Techniques , 2nd ed. By Greg T.Hermanson (Pierce Biotechnology, Thermo Fisher Scientific, Rockford, IL). Academic Press (an imprint of Elsevier): London, Amsterdam, Burlington, San Diego . 2008. ISBN 978-0-12-370501-3.
- 1. Sen Ji, Xiao Hu, et al. "Discovery of BT-114143, a Novel and Potent Phosphoric Acid-Containing Small-Molecule Plasminogen Activation Inhibitor for Hyperfibrinolysis." J Med Chem. 2025 Mar 27;68(6):6084-6099 PMID: 40099448
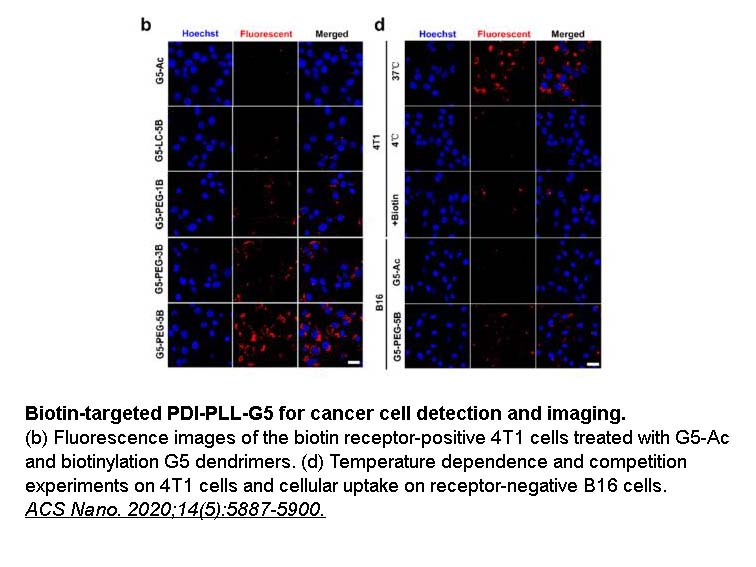
- 2. Cong H, Wang K, et al. "Tuning the Brightness and Photostability of Organic Dots for Multivalent Targeted Cancer Imaging and Surgery." ACS Nano. 2020;14(5):5887-5900. PMID:32356972
- 3. JIUHE ZHU. "The regulation of neuronal excitability by epilepsy-associated gene Nedd4-2." University of Illinois. Urbana-Champaign, 2019
- 4. Kamperman T, Koerselman M, et al. "Spatiotemporal material functionalization via competitive supramolecular complexation of avidin and biotin analogs." Nat Commun. 2019 Sep 25;10(1):4347. PMID:31554812
- 5. Zhang X, Qiu M, et al. "Autologous Red Blood Cell Delivery of Betamethasone Phosphate Sodium for Long Anti-Inflammation." Pharmaceutics. 2018 Dec 18;10(4). pii: E286. PMID:30567356
- 6. Zhu J, Lee KY, et al. "Epilepsy-associated gene Nedd4-2 mediates neuronal activity and seizure susceptibility through AMPA receptors." PLoS Genet. 2017 Feb 17;13(2):e1006634. PMID:28212375
• Protein labeling—biotinylate antibodies or other proteins for detection or purification using streptavidin probes or resins
• Intracellular labeling—membrane-permeable, can be used to label inside cells
• Amine-reactive—reacts with primary amines (-NH2), such as the side-chain of lysines (K) or the N-terminal-amine
• Irreversible—forms permanent amide bonds; spacer arm cannot be cleaved
• Solubility—water insoluble, must be dissolved in DMSO or DMF before further dilution in aqueous buffers
• Medium length—spacer arm is 22.4 angstroms; it consists of the biotin valeric acid group extended by a 6-atom chain
| Storage | Store at -20°C |
| M.Wt | 454.54 |
| Cas No. | 72040-63-2 |
| Formula | C20H30N4O6S |
| Synonyms | Succinimidyl 6-(biotinamido)hexanoate |
| Solubility | ≥22.73 mg/mL in DMSO; insoluble in EtOH; insoluble in H2O |
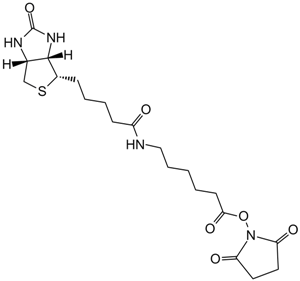
| Chemical Name | (2,5-dioxopyrrolidin-1-yl) 6-[5-[(3aS,4S,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]hexanoate |
| SDF | Download SDF |
| Canonical SMILES | O=C(CCCC[C@H]([C@H]1N2)SC[C@H]1NC2=O)NCCCCCC(ON(C(CC1)=O)C1=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Biotinylation method [1]: | |
|
Sample |
S. aureus |
|
Preparation method |
Soluble in DMSO or DMF. |
|
Reaction Conditions |
60 mg/ml, room temperature for 1 h. |
|
Applications |
Fixed cell suspensions of S. aureus were diluted to a 2% cell suspension in 0.2 M NaHCO3, pH 8.3. NHS-LC-biotin freshly dissolved in DMSO at a concentration of 60 mg/ml, was added to the cell suspension subsequently. After gentle tumble mixing for 60min at room temperature, the reaction was stopped by removal of excess biotin via gel filtration over a PD-10 column and washed extensively in 0.01 mM Tris-buffered 0.15 M NaCl (TBS). Successful protein biotinylation was examining their reactivity with alkaline phosphatase-conjugated streptavidin(AP-STRAV). AP-STRAV binding was detected as a function of pNPP hydrolysis. |
|
References: [1]. TRUC NGUYEN, BERHANE GHEBREHIWET, AND ELLINOR I. B. PEERSCHKE. Staphylococcus aureus Protein A Recognizes Platelet gC1qR/p33: a Novel Mechanism for Staphylococcal Interactions with Platelets. INFECTION AND IMMUNITY 2000, p. 2061–2068. |
|
| Description | NHS-LC-Biotin is a amine-reactive biotinylation agent | |||||
| Targets | ||||||
| IC50 | ||||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
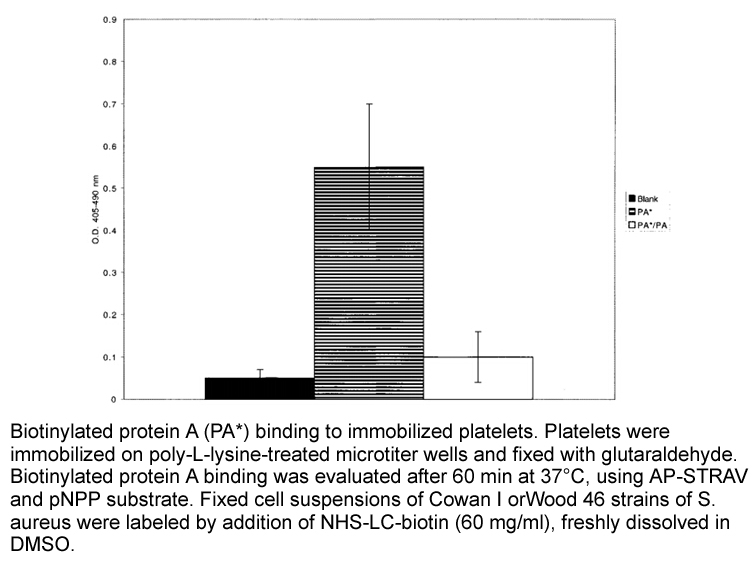
Related Biological Data
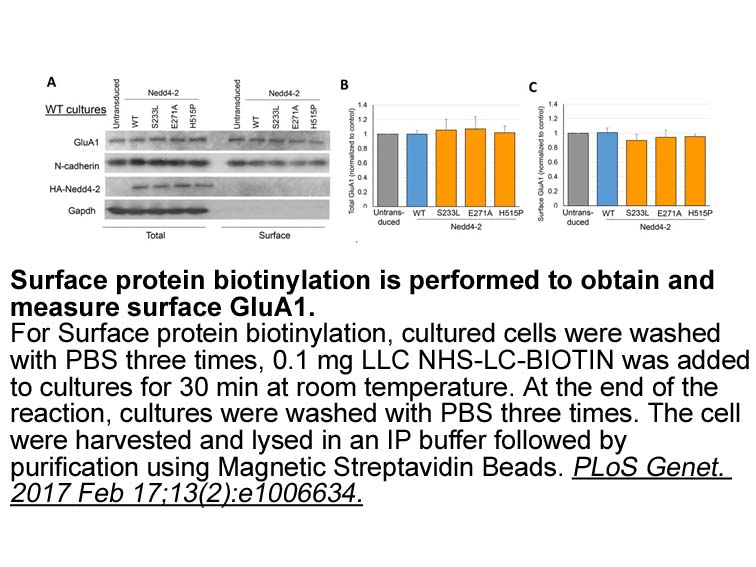
Related Biological Data
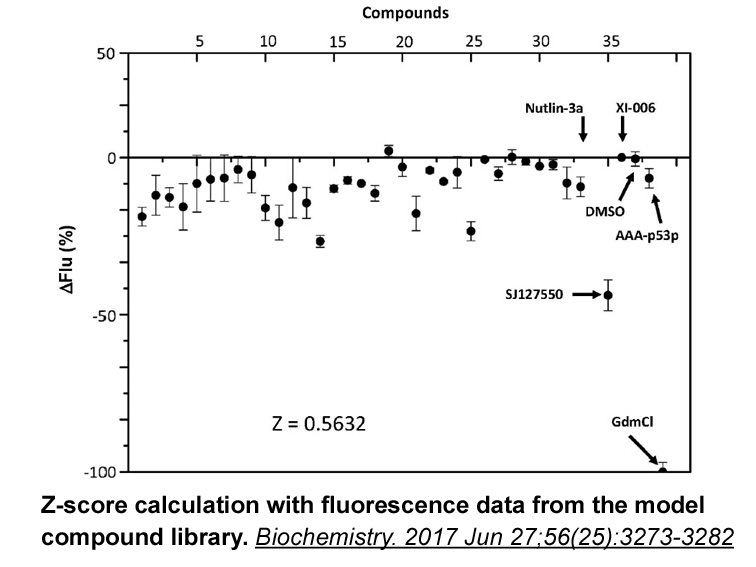
Related Biological Data
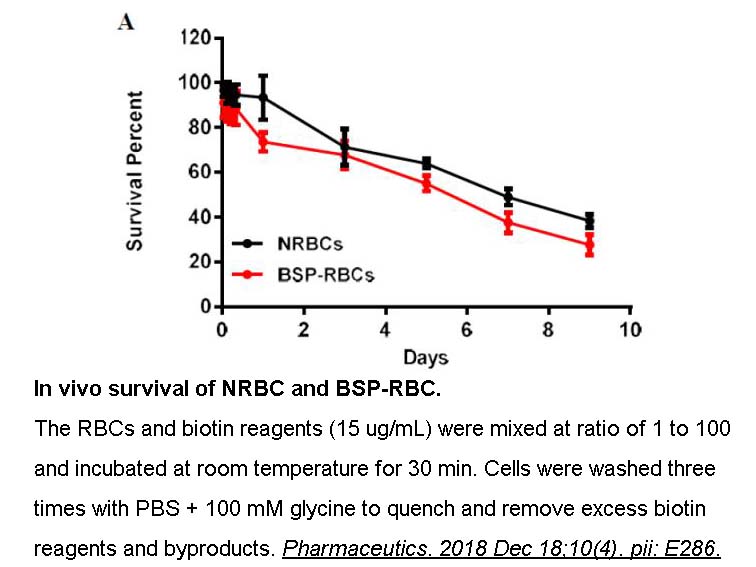
Related Biological Data
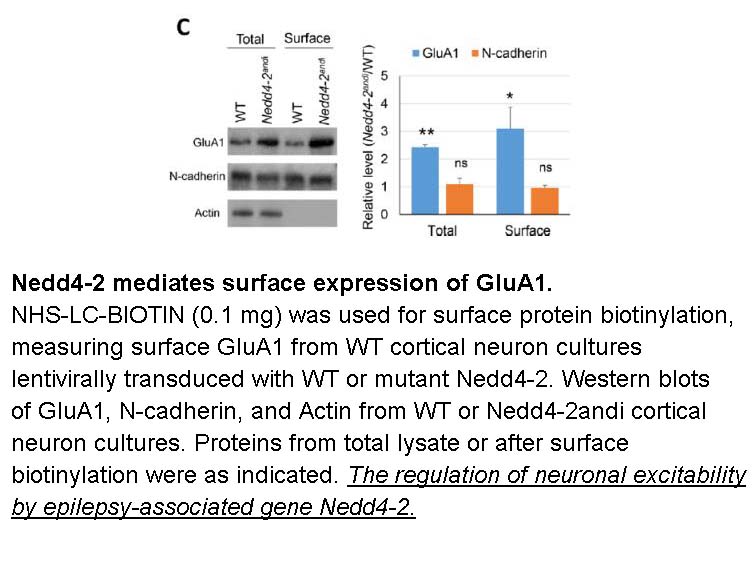
Related Biological Data