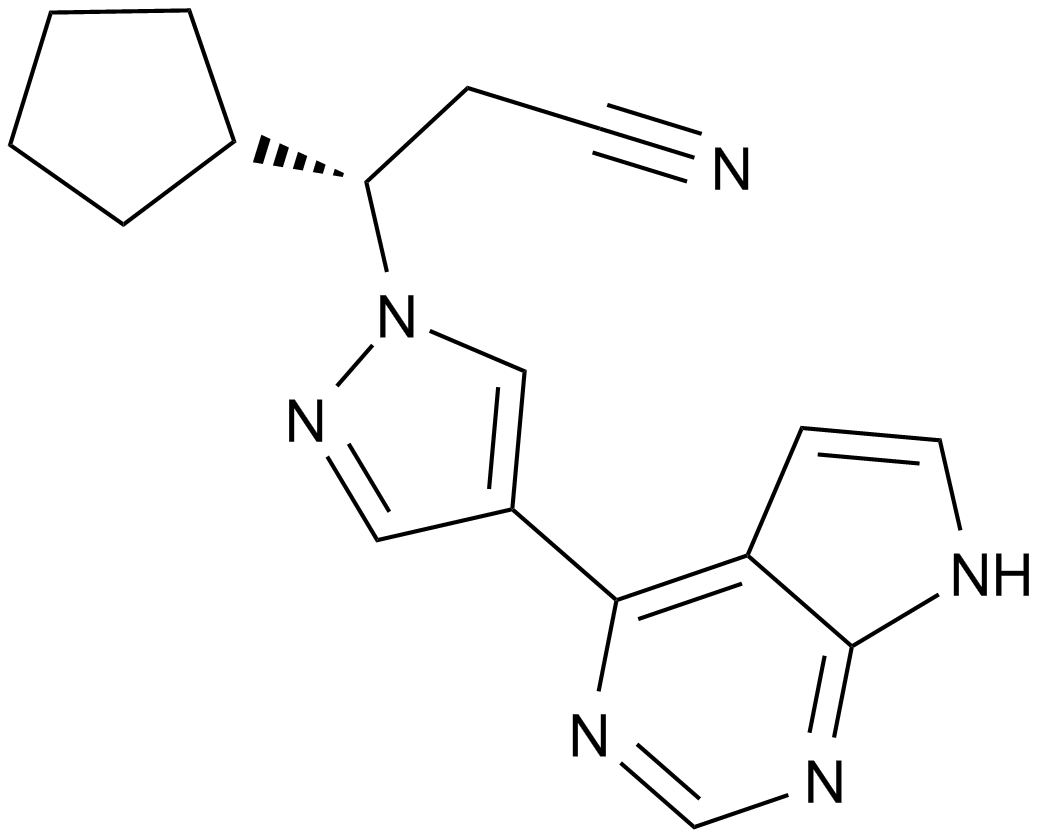
Ruxolitinib (INCB018424)
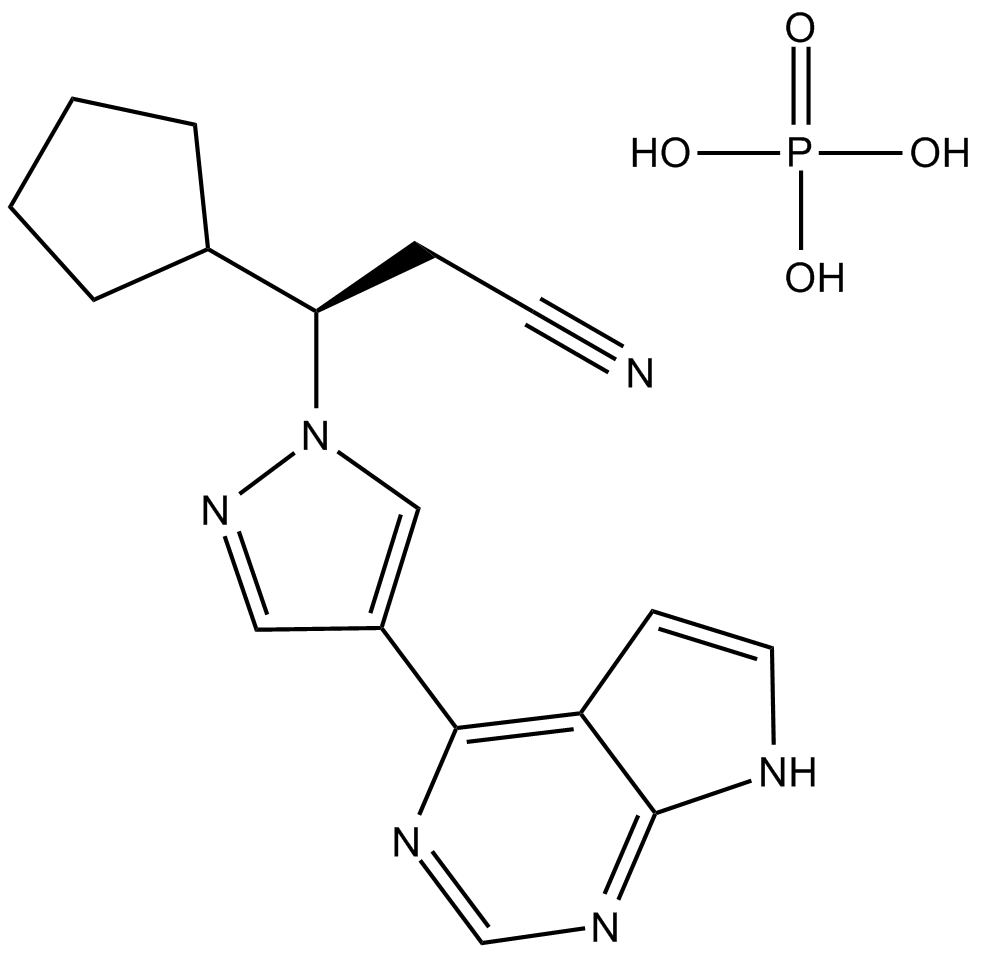
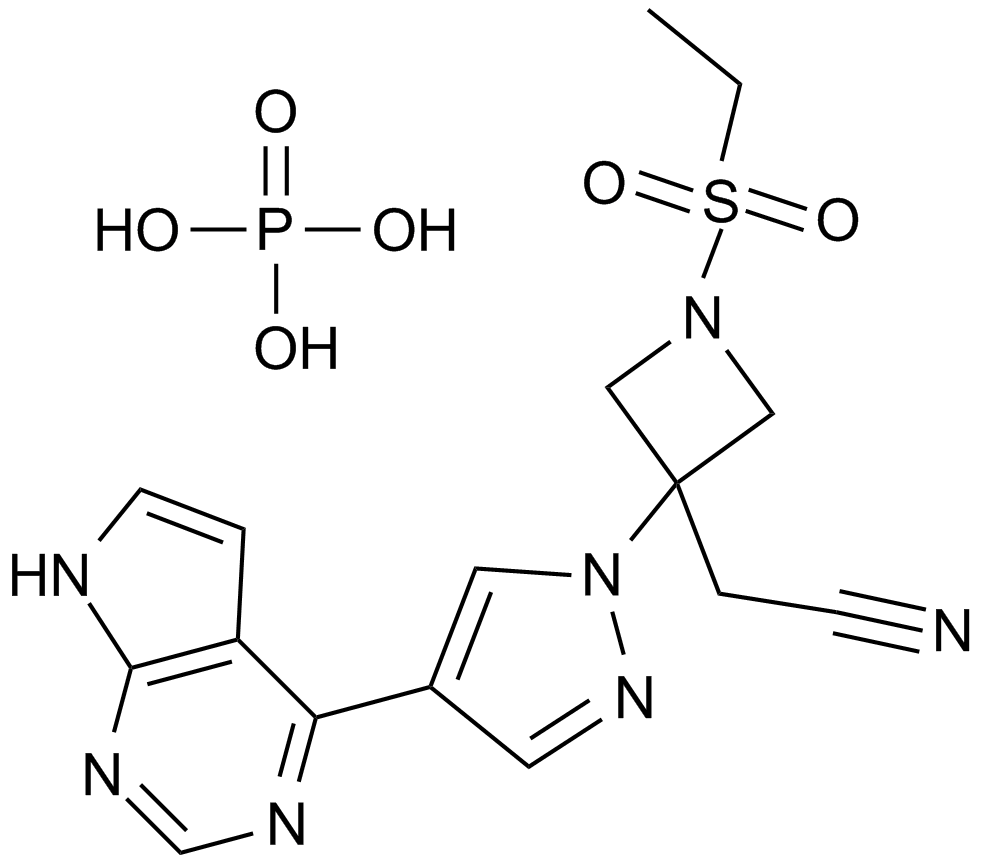
Ruxolitinib dihydrochloride is a specific inhibitor of Janus-associated kinase (JAK1 and JAK2). Ruxolitinib is a small molecular with the formula of C17H21N6O4Pand Molecular Weight of 404. Ruxolitinib phosphate is an administered ATP-competitive cyclopentylpropionitrile derivative and shows inhibition activity on JAK1 and JAK2. Ruxolitinib inhibits phosphorylation of JAK1/2, STAT5, and ERK1/2, resulting in reduced cellular proliferation.
References
1. Ruxolitinib inhibits transforming JAK2 fusion proteins in vitro and induces complete cytogenetic remission in t (8; 9)(p22; p24)/PCM1-JAK2–positive chronic. E Lierman, D Selleslag, S Smits, J Billiet. Blood. 2012
2. Ruxolitinib for the treatment of myelofibrosis. A Ostojic, R Vrhovac, S Verstovsek. Ther Clin Risk Manag. 2011
- 1. Boshen Yang, Taixi Li, et al. "Ruxolitinib-based senomorphic therapy mitigates cardiomyocyte senescence in septic cardiomyopathy by inhibiting the JAK2/STAT3 signaling pathway." Int J Biol Sci. 2024 Aug 12;20(11):4314-4340 PMID: 39247818
- 2. Alexandra Hardy, Siddharth Bakshi, et al. "The timing and magnitude of the type I interferon response are correlated with disease tolerance in arbovirus infection." mBio. 2023 Jun 27;14(3):e0010123. PMID: 37097030
- 3. Lijuan Huang, Yanjiao Zhang, et al. "Ferrostatin-1 Polarizes Microglial Cells Toward M2 Phenotype to Alleviate Inflammation After Intracerebral Hemorrhage." Neurocrit Care. 2022 Jan 31. PMID: 35099711
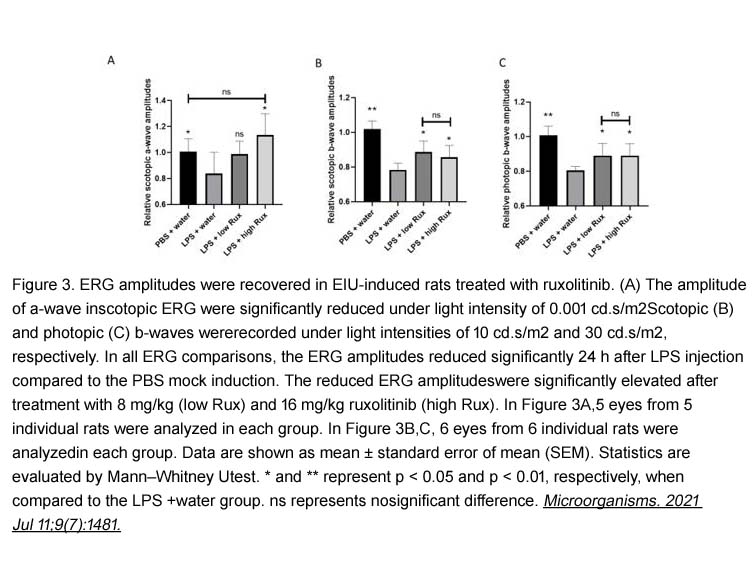
- 4. Lin Du, YolandaWong Ying Yip, et al. "Ruxolitinib Alleviates Uveitis Caused by Salmonella typhimurium Endotoxin." Microorganisms. 2021 Jul 11;9(7):1481. PMID:34361917
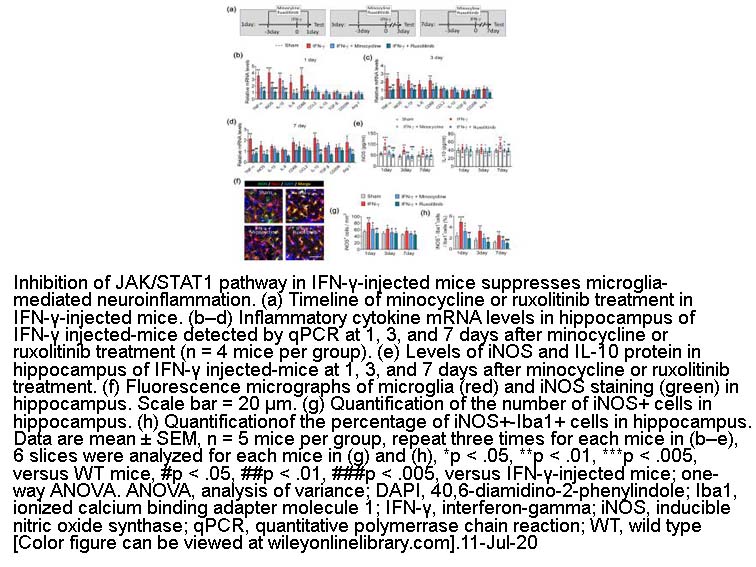
- 5. Jinqiang Zhang, Hui He, et al. "Priming of microglia with IFN ‐γ impairs adult hippocampal neurogenesis and leads to depression‐like behaviors and cognitive defects." Glia. 2020 Dec;68(12):2674-2692. PMID:32652855
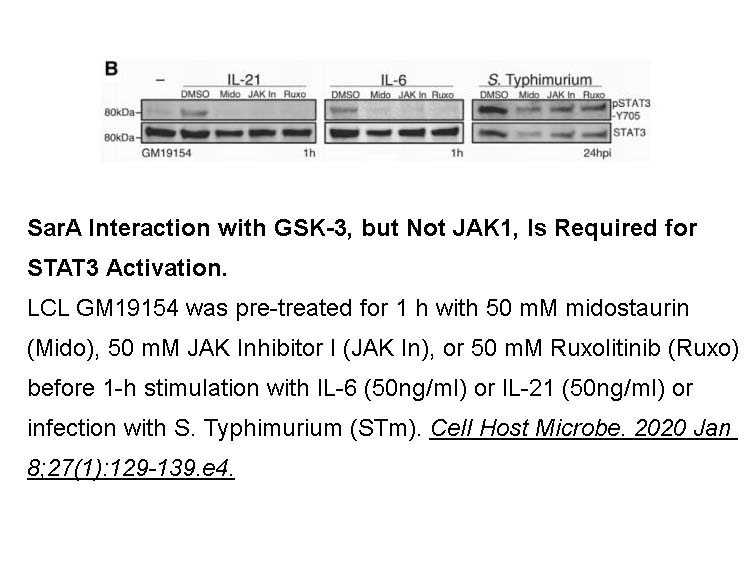
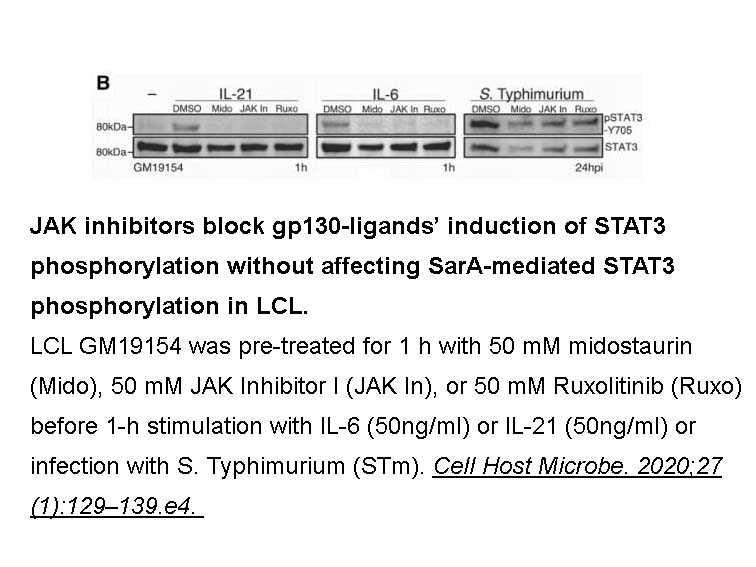
- 6. Gibbs KD, Washington EJ, et al. "The Salmonella Secreted Effector SarA/SteE Mimics Cytokine Receptor Signaling to Activate STAT3." Cell Host Microbe. 2020 Jan 8;27(1):129-139.e4. PMID:31901521
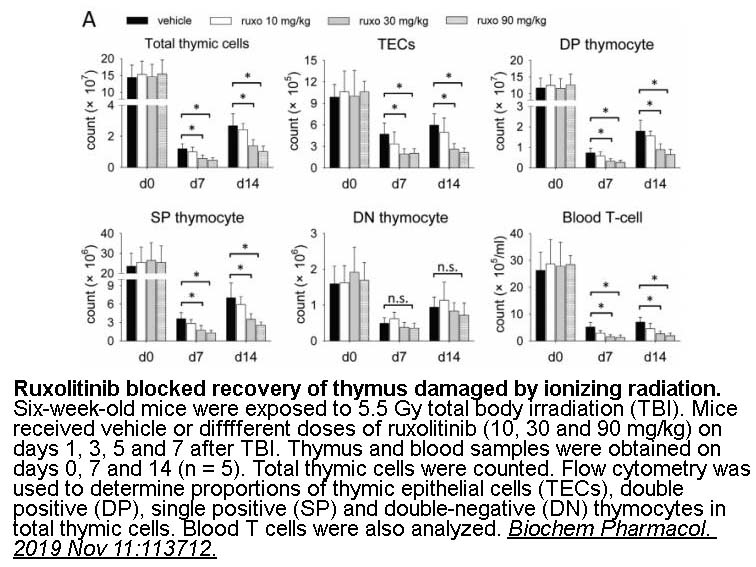
- 7. Li L, Shang L, et al. "Janus kinase inhibitor ruxolitinib blocks thymic regeneration after acute thymus injury." Biochem Pharmacol. 2019 Nov 11:113712. PMID:31726048
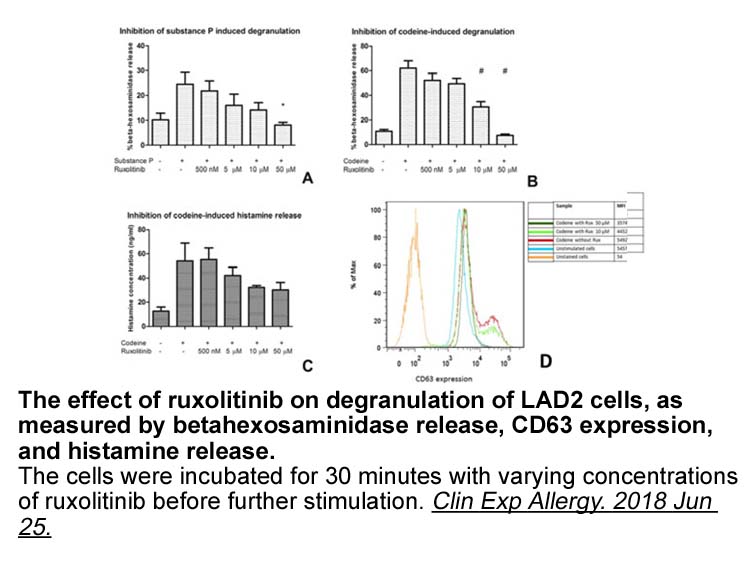
- 8. Hermans MAW, Schrijver B, et al. "The JAK1/JAK2- inhibitor ruxolitinib inhibits mast cell degranulation and cytokine release." Clin Exp Allergy. 2018 Jun 25. PMID:29939445
- 9. Zhang S, Li Z, et al. "Interleukin-4 Enhances the Sensitivity of Human Monocytes to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Through Upregulation of Death Receptor 4." J Interferon Cytokine Res. 2018 Apr;38(4):186-194. PMID:29638207
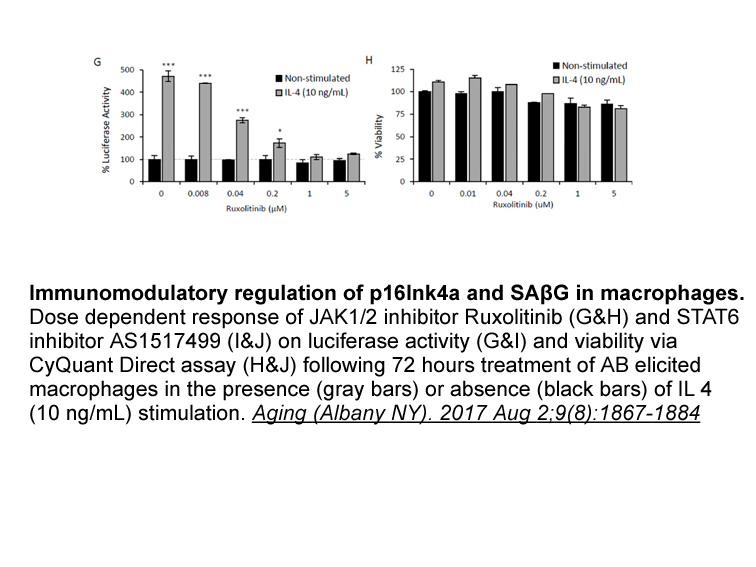
- 10. Hall BM, Balan V, et al. "p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli." Aging (Albany NY). 2017 Aug 2;9(8):1867-1884. PMID:28768895
- 11. Radhakrishnan H, Ilm K, et al. "MACC1 regulates Fas mediated apoptosis through STAT1/3-Mcl-1 signaling in solid cancers." Cancer Lett. 2017 Jun 23. pii: S0304-3835(17)30402-0. PMID:28649004
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 306.37 |
| Cas No. | 941678-49-5 |
| Formula | C17H18N6 |
| Synonyms | Ruxolitinib, INCB018424, INCB-018424 |
| Solubility | insoluble in H2O; ≥15.32 mg/mL in DMSO; ≥17.53 mg/mL in EtOH |
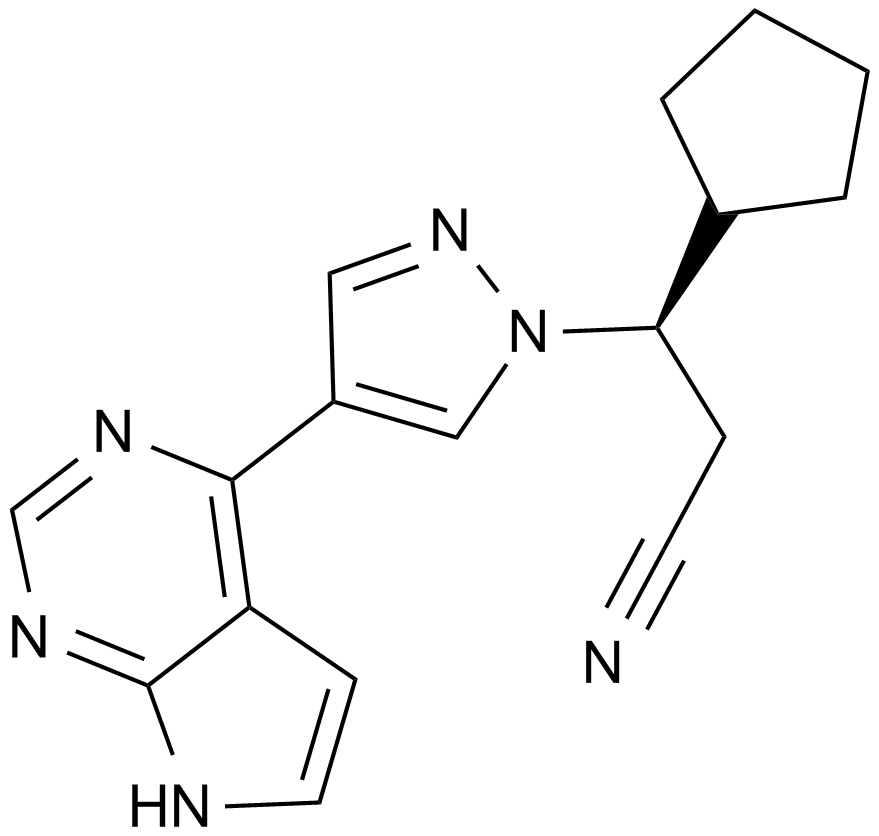
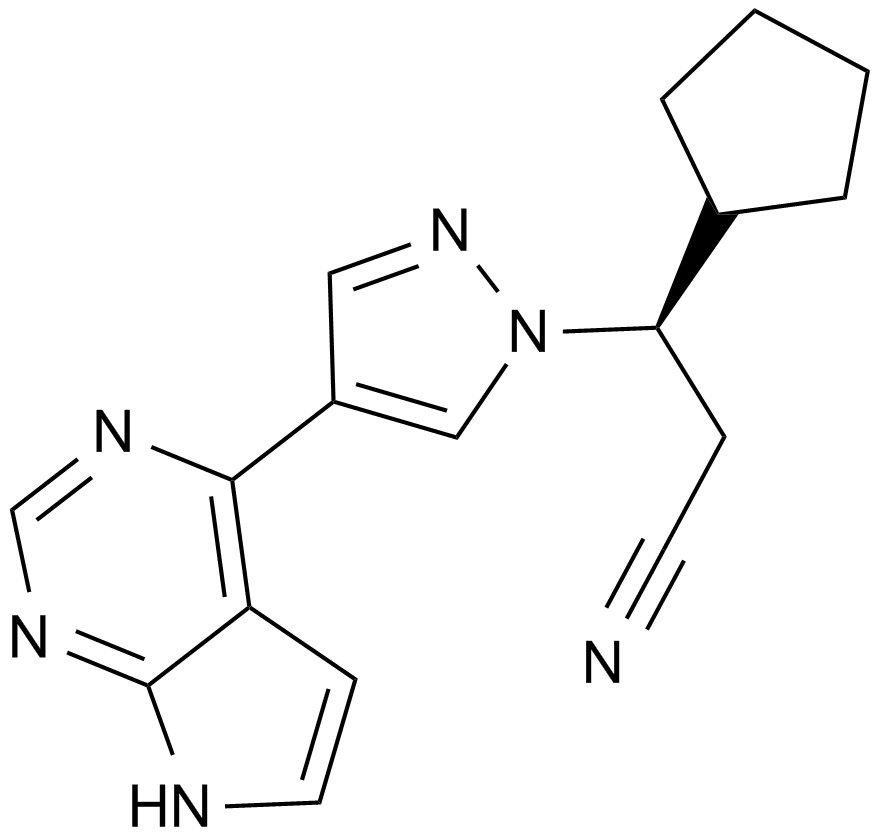
| Chemical Name | (3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propanenitrile |
| SDF | Download SDF |
| Canonical SMILES | C1CCC(C1)C(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment: [1] | |
|
Cell lines |
Primary mononuclear cells isolated from patients with PV or normal control persons |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
IC50: erythroid progenitors: 407 nM for normal donors, 223 nM for PV donors myeloid progenitors: 511 nM for normal donors, 444 nM for PV donors 14 days |
|
Applications |
Growth of clonogenic progenitors of erythroid (BFU-E) and myeloid origin (CFU-M) was assessed in colony-forming assays in the presence of increasing concentrations of INCB018424. Dose-dependent inhibition of the growth of erythroid and myeloid progenitors was observed with INCB018424. The mean IC50 for INCB018424 against erythroid progenitors was 407 nM for normal donors and 223 nM for PV donors. A similar effect was observed on myeloid progenitors (CFU-M), with IC50 values of 511 nM and 444 nM for control and PV samples, respectively. |
| Animal experiment: [2] | |
|
Animal models |
C57BL/6N mice |
|
Dosage form |
Oral administration, 75 mg/kg |
|
Applications |
Mice receiving 75 mg/kg ruxolitinib or vehicle 6 hours prior to and 6 hours after injection of OVA/CpG were analyzed for expression of activation markers on CD11c 1CD81 splenic DCs. Lower expression levels of CD40, CD80, CD86 as well as MHC I and II molecules were detected in ruxolitinib-challenged animals. Next, ruxolitinib or vehicle was fed to mice 6 hours prior to as well as 6 hours and 18 hours after priming with OVA/CpG and adoptive transfer of CFSE-labeled OT-I cells. Analysis of transferred CFSE-labeled OT-I T cells revealed reduced proliferation, CD25 expression, and IFN-production in mice pretreated with ruxolitinib. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood, 2010, 115(15): 3109-3117. [2] Heine A, Held S A E, Daecke S N, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood, 2013, 122(7): 1192-1202. |
|
| Description | INCB018424 is the first potent, selective inhibitor of JAK1/2 to enter the clinic with IC50 of 3.3 nM/2.8 nM, >130 fold selectivity for JAK1/2 versus JAK3. | |||||
| Targets | JAK1 | JAK2 | ||||
| IC50 | 3.3 nM | 2.8 nM | ||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data