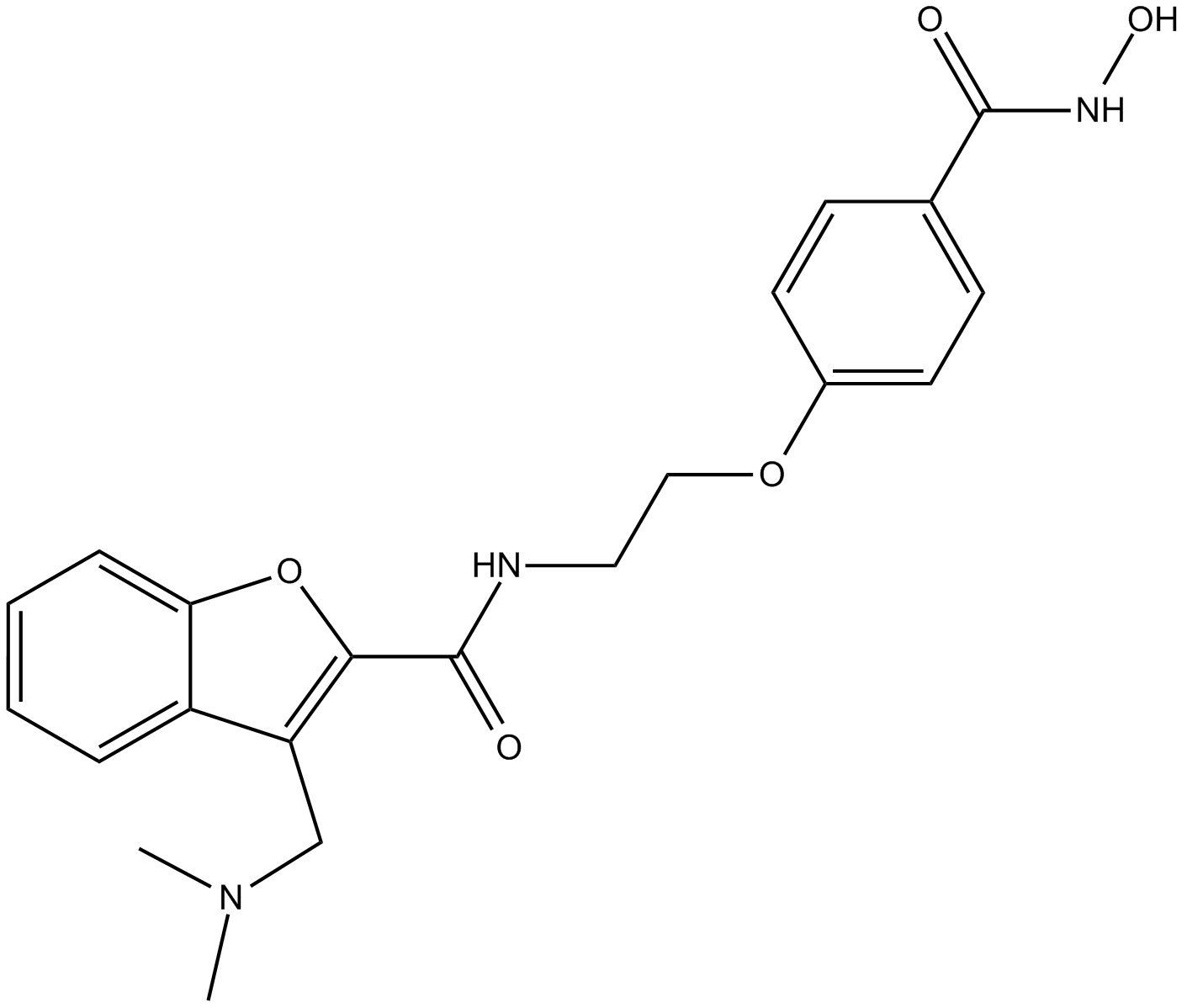
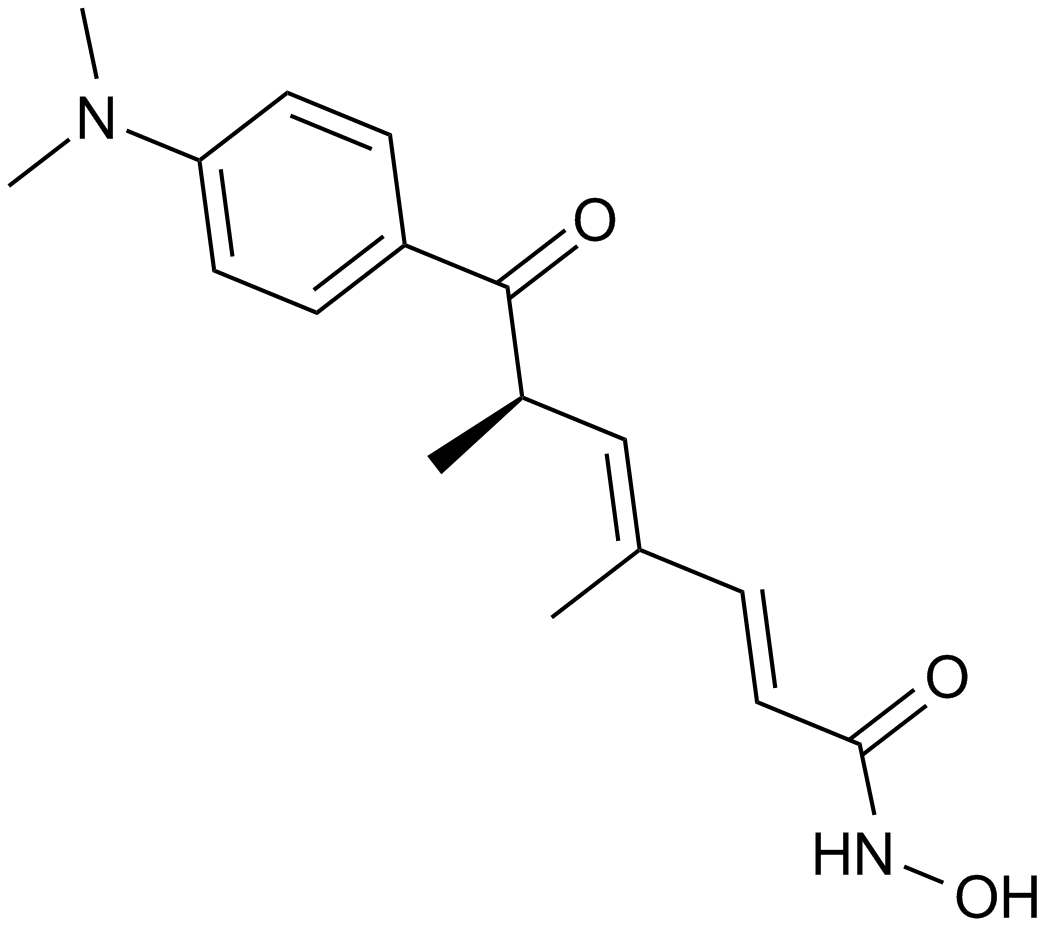
Panobinostat (LBH589)
Panobinostat, as known as LBH589, is a novel and potent hydroxamic acid-based deacetylase inhibitor (DACis)that inhibits a broad spectrum of histone deacetylases (HDACs), including all Classes 1, 2 and 4 HDAC enzymes, at low nanomolar concentrations. According to previous studies, it not only induces apoptosis in multiple myeloma (MM) cells via caspase activation and poly(ADP-ribose) polymerase (PARP) cleavage, but also induces potent cell growth inhibition, cell-cycle arrest, and apoptosis in a time- and dose-dependent manner in both Philadelphia chromosome-negative (Ph-) actue lymphoblastic leukemia (ALL) cells lines (T-cell MOLT-4 and pre-B-cell Reh), which are correlated with induction of histone (H3K9 and H4K8) hyperacetylation, activation of p21 and p27, and suppression of c-Myc.
Reference
Wenlin Shao, Joseph D. Growney, Yun Feng, Gregory O’Connor, Minying Pu, Wenjing Zhu, Yung-Mae Yao, Paul Kwon, Stephen Fawell and Peter Atadja. Activity of deacetylase inhibitor panobinostat (LBH589) in cutaneous T-cell lymphoma models: defining molecular mechanisms of resistance. Int. J. Cancer: 127, 2199-2208 (2010)
Laurence Catley, Ellen Weisberg, Tanyel Kiziltepe, Yu-Tzu Tai, Teru Hideshima, Paola Neri, Pierfrancesco Tassone, Peter Atadja, Dharminder Chauhan, Nikhil C. Munshi and Keneth C. Anderson. Aggresome induction by proteasome inhibitor bortezomib and α-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood (2006); 108(10): 3441-3449
Anna Scuto, Mark Kirschbaum, Claudia Kowolik, Leo Kretzner, Agnes Juhasz, Peter Atadja, Vinod Pullarkat, Ravi Bhatia, Stephen Forman, Yun Yen, and Richard Jove. The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells. Blood (2008); 111(10):5093-5100
- 1. Pulak R. Manna, Deborah Molehin, et al. "Acetylation of Steroidogenic Acute Regulatory Protein Sensitizes 17β-Estradiol Regulation in Hormone-Sensitive Breast Cancer Cells" Int. J. Mol. Sci. 2024, 25(16), 8732
- 2. Robiya Joseph, et al. "EphA2-and HDAC-Targeted Combination Therapy in Endometrial Cancer." Int J Mol Sci. 2024 Jan 20;25(2):1278. PMID: 38279277
- 3. Manna PR, Ramachandran S, et al. "Expression and Function of StAR in Cancerous and Non-Cancerous Human and Mouse Breast Tissues: New Insights into Diagnosis and Treatment of Hormone-Sensitive Breast Cancer." Int J Mol Sci 2023 Jan 01;24(1) PMID: 36614200
- 4. Joseph Boyle, Derick Chiappo, et al. "Aminocoumarin-based heme oxygenase activity fluorescence probe reveals novel aspects of HO-1 regulation." Research Square. 22 Nov, 2023.
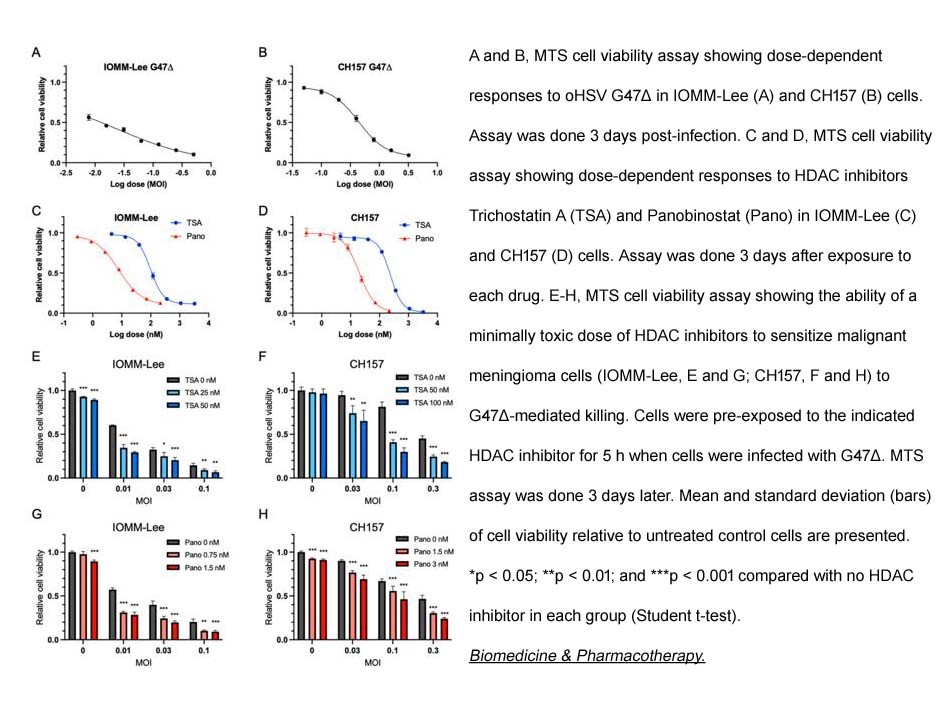
- 5. Yoichiro Kawamura, Lingyang Hua, et al. "Histone deacetylase inhibitors enhance oncolytic herpes simplex virus therapy for malignant meningioma." Biomed Pharmacother. 2022 Nov;155:113843. PMID: 36271587
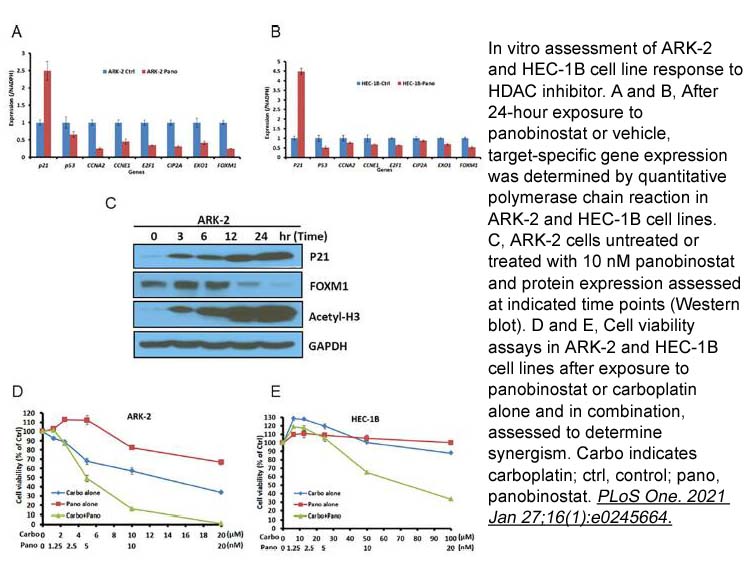
- 6. Jesus Gonzalez Bosquet, Qing Zhang, et al. "Association of a novel endometrial cancer biomarker panel with prognostic risk, platinum insensitivity, and targetable therapeutic options." PLoS One. 2021 Jan 27;16(1):e0245664. PMID: 33503056
- 7. Susanna Nencetti, Doretta Cuffaro, et al. "Identification of histone deacetylase inhibitors with (arylidene) aminoxy scaffold active in uveal melanoma cell lines." J Enzyme Inhib Med Chem. 2021 Dec;36(1):34-47. PMID: 33100043
- 8. Sauradip Chaudhuri, Martha Fowler, et al. "β-Cyclodextrin-Poly (β-Amino Ester) Nanoparticles Are a Generalizable Strategy for High Loading and Sustained Release of HDAC Inhibitors." ChemRxiv. Mar 27. 2020.
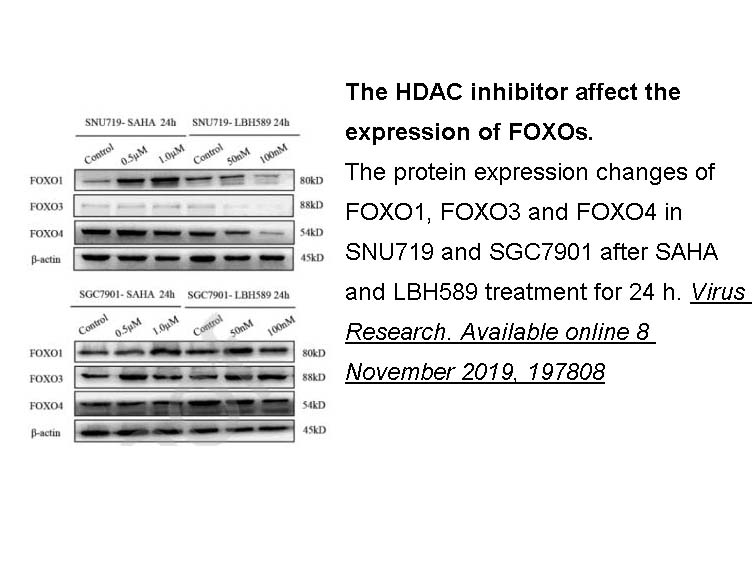
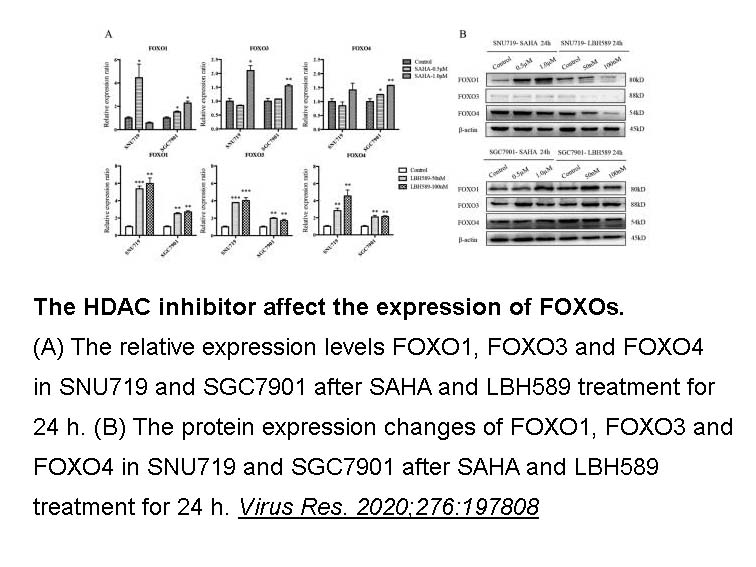
- 9. Liu W, Song YY, et al. "Dysregulation of FOXO transcription factors in Epstein-Barr virus-associated gastric carcinoma." Virus Res. 2020;276:197808. PMID: 31712122
- 10. Martins VF, Begur M, et al. "Acute inhibition of protein deacetylases does not impact skeletal muscle insulin action." Am J Physiol Cell Physiol. 2019 Aug 28. PMID: 31461343
- 11. Simic D, Sang N. "Compounds targeting class II histone deacetylases do not cause panHDACI-associated impairment of megakaryocyte differentiation." Exp Hematol. 2019 Jan 4. pii: S0301-472X(19)30005-0. PMID: 30611870
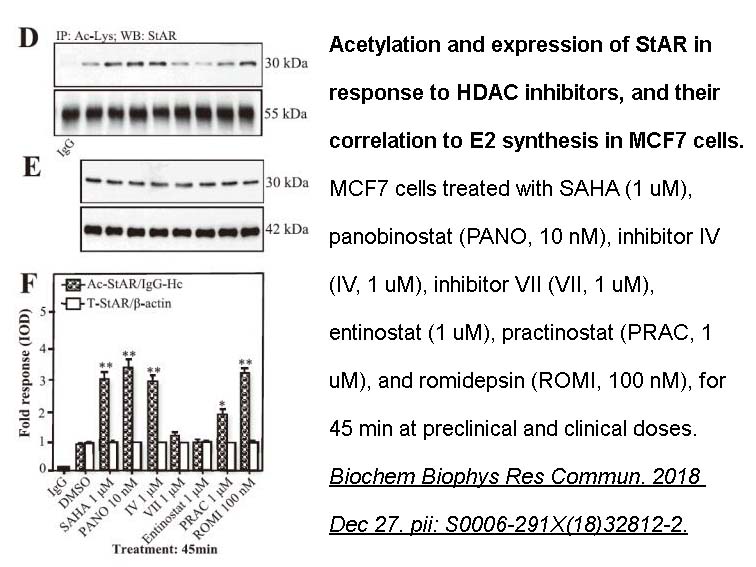
- 12. Manna PR, Ahmed AU, et al. "Overexpression of the steroidogenic acute regulatory protein in breast cancer: Regulation by histone deacetylase inhibition." Biochem Biophys Res Commun. 2019 Feb 5;509(2):476-482. PMID: 30595381
- 13. Hacker KE, Bolland DE, et al. "The DEK Oncoprotein Functions in Ovarian Cancer Growth and Survival." Neoplasia. 2018 Dec;20(12):1209-1218. PMID: 30412857
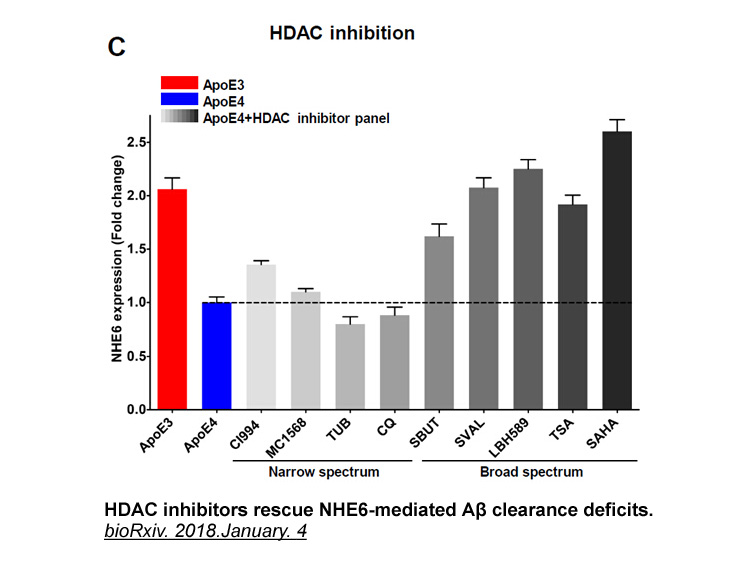
- 14. Hari Prasad, Rajini Rao. "The Amyloid Clearance Defect in ApoE4 Astrocytes is Corrected by Epigenetic Restoration of NHE6." bioRxiv. 2018.January. 4.
- 15. Lee HM, Lee E, et al. "Drug repurposing screening identifies bortezomib and panobinostat as drugs targeting cancer associated fibroblasts (CAFs) by synergistic induction of apoptosis." Invest New Drugs. 2018 Jan 18. PMID: 29349597
- 16. Ballante F, Reddy DR, et al. "Structural insights of SmKDAC8 inhibitors: Targeting Schistosoma epigenetics through a combined structure-based 3D QSAR, in vitro and synthesis strategy." Bioorg Med Chem. 2017 Apr 1;25(7):2105-2132. PMID: 28259528
- 17. Bagnall NH, Hines BM, et al."Insecticidal activities of histone deacetylase inhibitors against a dipteran parasite of sheep, Lucilia cuprina." Int J Parasitol Drugs Drug Resist. 2017 Apr;7(1):51-60. PMID: 28110187
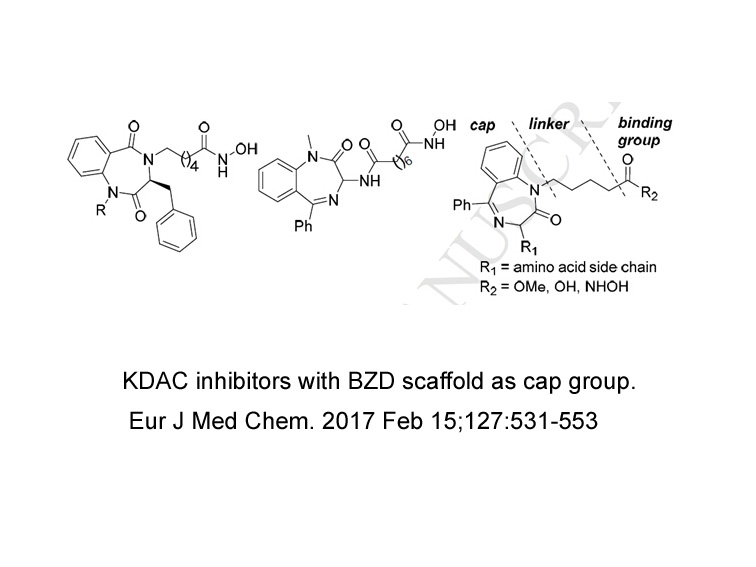
- 18. Reddy DR, Ballante F, et al. "Design and synthesis of benzodiazepine analogs as isoform-selective human lysine deacetylase inhibitors." Eur J Med Chem. 2017 Feb 15;127:531-553. PMID: 28109947
- 19. Chatterjee N, Tian M, et al. "Keap1-Independent Regulation of Nrf2 Activity by Protein Acetylation and a BET Bromodomain Protein."PLoS Genet. 2016 May 27;12(5):e1006072. PMID: 27233051
- 20. Reddy DN, Ballante F, et al. "Design and Synthesis of Simplified Largazole Analogues as Isoform-Selective Human Lysine Deacetylase Inhibitors." J Med Chem. 2016 Feb 25;59(4):1613-33. PMID: 26681404
| Storage | Store at -20°C |
| M.Wt | 349.43 |
| Cas No. | 404950-80-7 |
| Formula | C21H23N3O2 |
| Synonyms | Panobinostat,LBH589,LBH-589, Faridak, NVP-LBH589, |
| Solubility | insoluble in H2O; insoluble in EtOH; ≥17.47 mg/mL in DMSO |
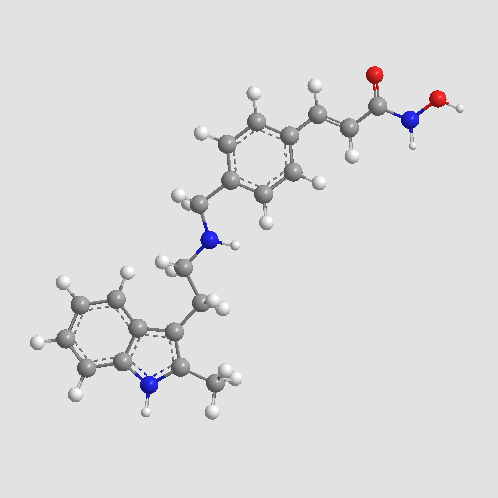
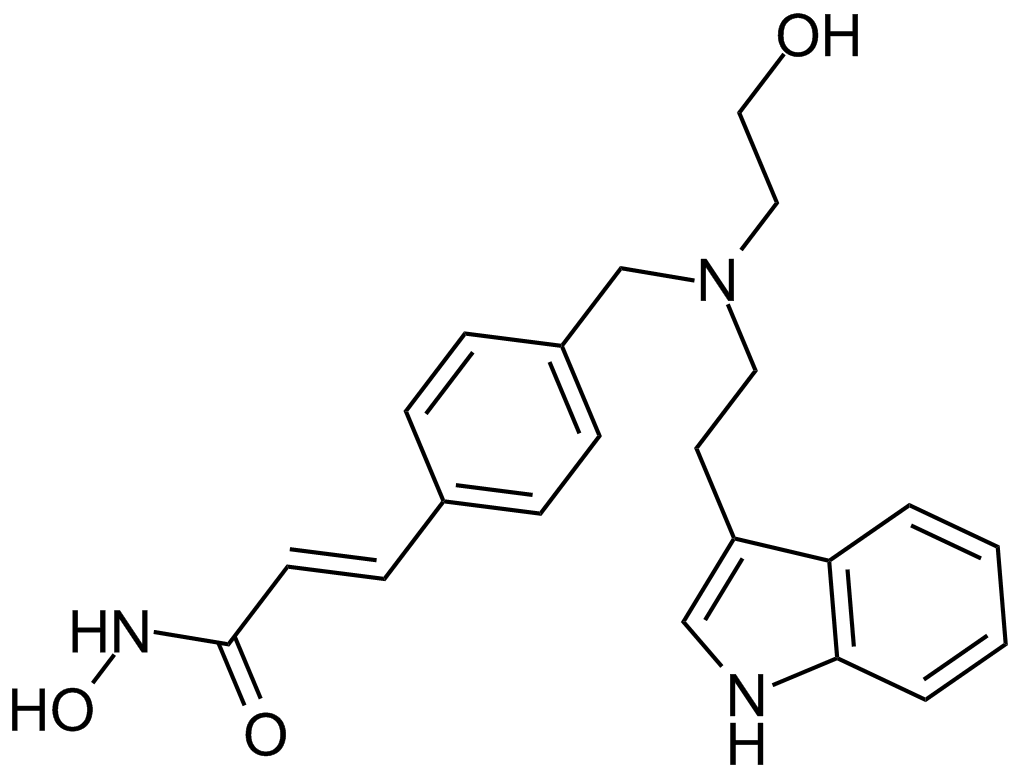
| Chemical Name | (E)-N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]prop-2-enamide |
| SDF | Download SDF |
| Canonical SMILES | CC1=C(C2=CC=CC=C2N1)CCNCC3=CC=C(C=C3)C=CC(=O)NO |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
MCF-7aro, LTEDaro, Exe-R, Let-R, Ana-R cell lins |
|
Preparation method |
The solubility of this compound in DMSO is |
|
Reaction Conditions |
6d; 20 nM |
|
Applications |
To study cellular response to AIs and the mechanisms of acquired AI resistance, we used the previously generated AI-responsive cell line MCF-7aro and AI-resistant variants of MCF-7aro created following in vitro selection against each AI (i.e., Exe-R, Let-R, and Ana-R) or long-term culture in the absence of estrogen (i.e., LTEDaro). MCF-7aro, LTEDaro and three AI-resistant cell lines were exposed to increasing concentrations of LBH589. This drug-inhibited proliferation of all cell lines in a dose-dependent manner. |
| Animal experiment [1]: | |
|
Animal models |
Female, 6- to 7-week-old ovariectomized, BALB/c Nu–Nu athymic mice |
|
Dosage form |
20 mg/kg, three times per week, intraperitoneal injection |
|
Applications |
To evaluate the inhibitory effects of LBH589 on AI resistance in vivo, we used the exemestane-resistant MCF7aro xenograft model. LBH589 treatment significantly inhibited the growth of exemestane-resistant tumors; tumor weight at the end of experiment was significantly lesser in mice treated with LBH589 than in control mice. No mice in the LBH589 treat-ment groups showed significant body weight loss indicating that the LBH589 treatment was well tolerated. Consistent with the effect of LBH589 on gross character-istics of the tumors, proliferation (assessed by Ki-67 staining) of tumor cells was significantly decreased in LBH589-treated mice and apoptosis (assessed by staining for cleaved PARP) of tumor cells was significantly increased. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Kubo M, Kanaya N, Petrossian K, et al. Inhibition of the proliferation of acquired aromatase inhibitor-resistant breast cancer cells by histone deacetylase inhibitor LBH589 (panobinostat)[J]. Breast cancer research and treatment, 2013, 137(1): 93-107. |
|
| Description | Panobinostat (LBH589) is a novel broad-spectrum inhibitor of HDAC with IC50 of 5 nM. | |||||
| Targets | HDAC (MOLT-4 cells) | HDAC (Reh cells) | ||||
| IC50 | 5 nM | 20 nM | ||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data