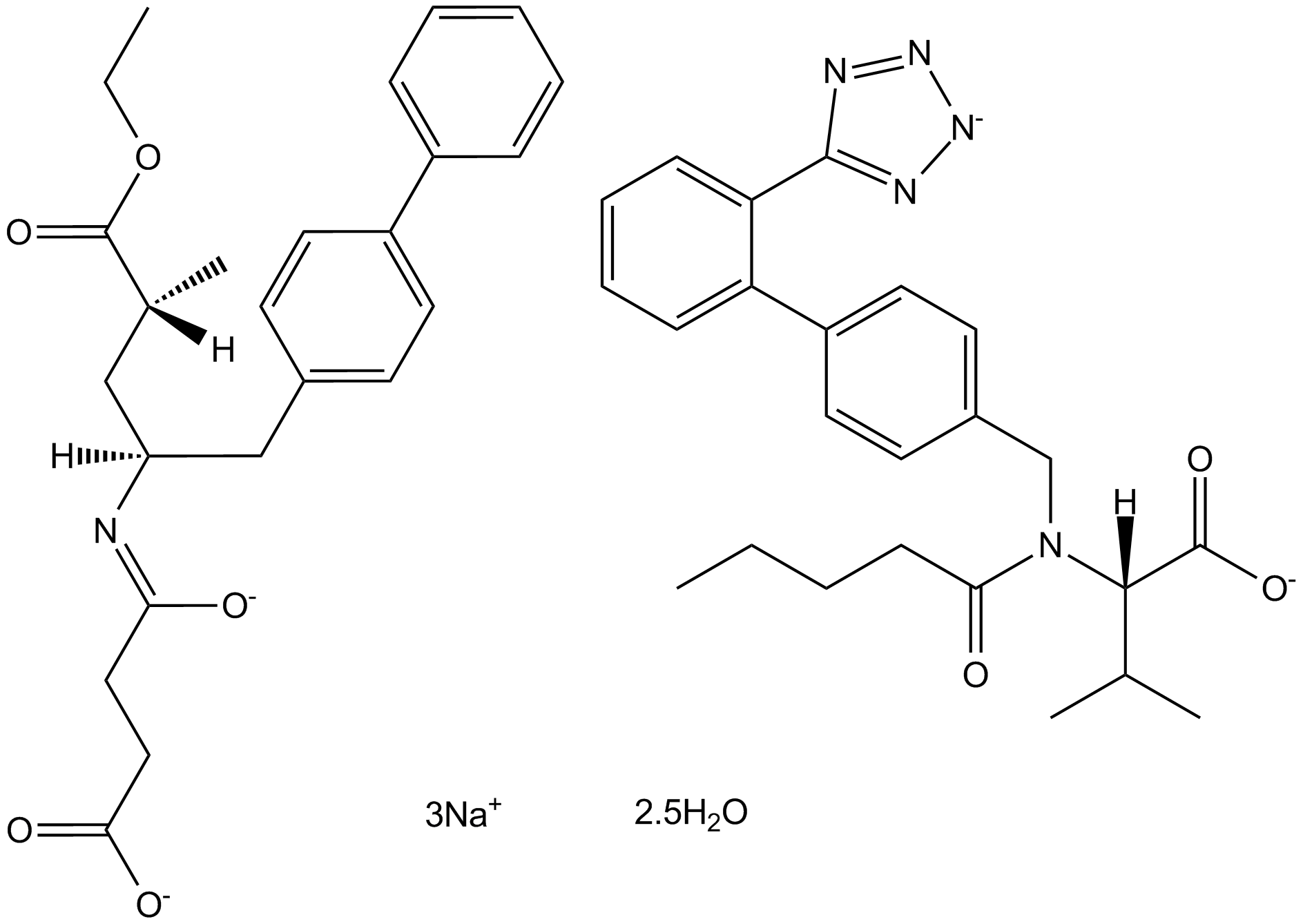
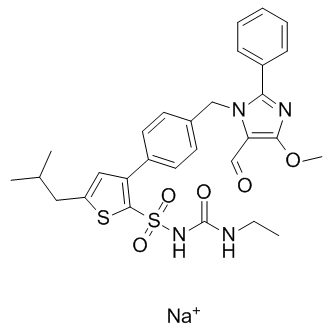
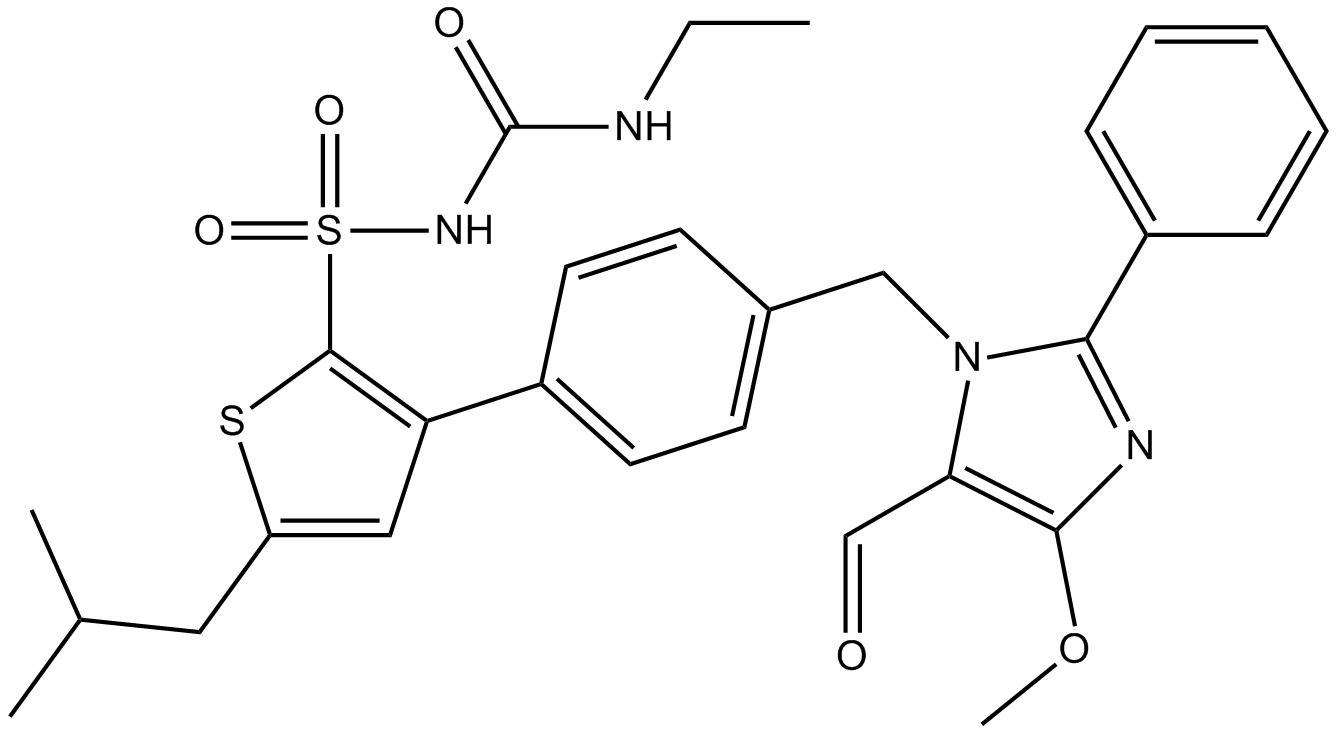
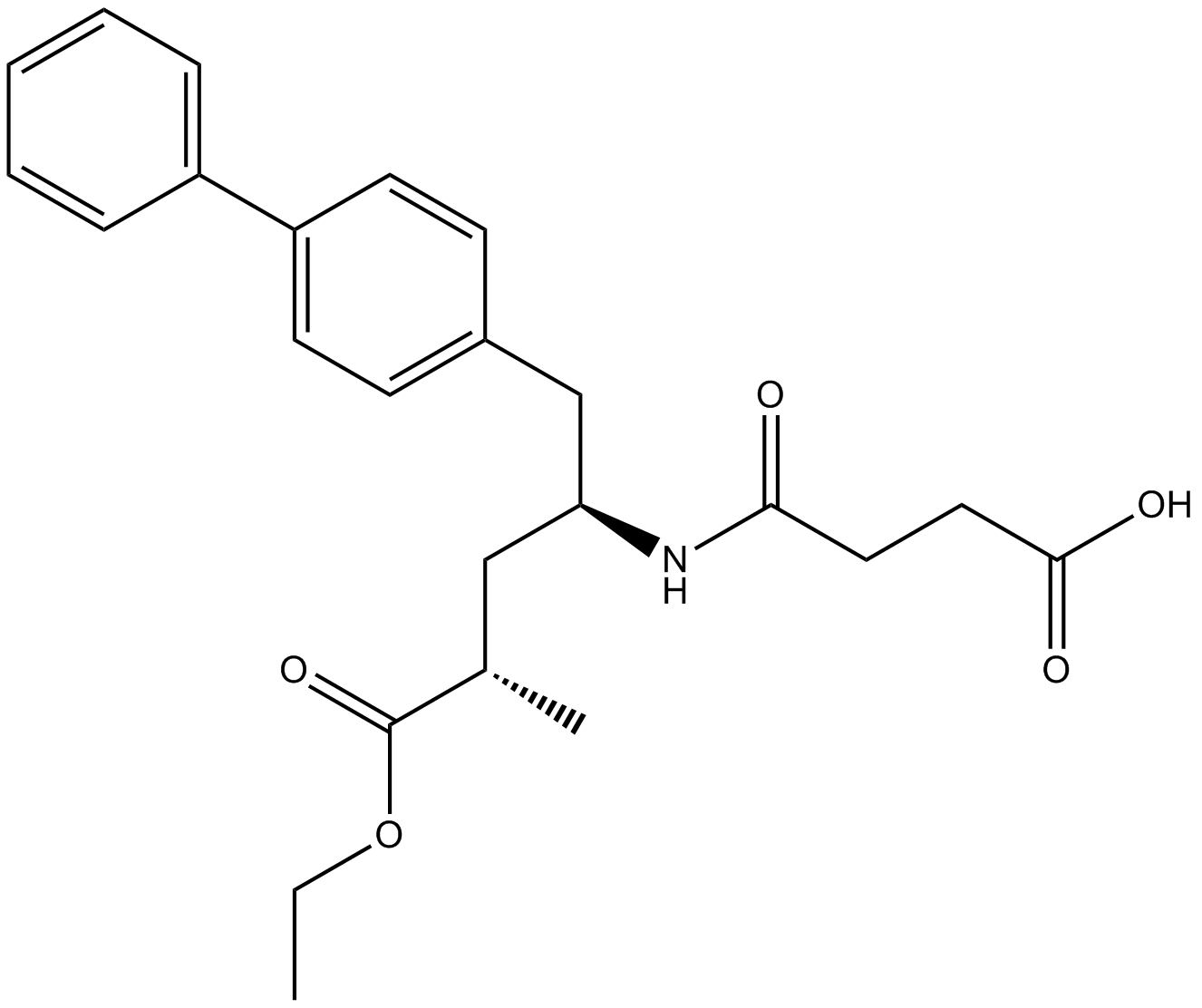
LCZ696
LCZ696 is a first in class ARNi (angiotensin receptor neprilysin inhibitor) comprising anionic moieties of AR valsartan and the neprilysin inhibitor prodrug AHU377 (1:1 ratio) for heart failure and hypertension.
The angiotensin receptors are G-protein-coupled receptors. They mediate the cardiovascular and other effects of angiotensin II which is a bioactive peptide of the renin–angiotensin system. Neprilysin is a neutral endopeptidase that degrades endogenous vasoactive peptides such as natriuretic peptides. Inhibition of neprilysin increases the natriuretic peptides concentration that contributed to cardiac, vascular and renal protection. [1]
In Sprague-Dawley rats, oral administration of LCZ696 led to a dose-dependent rise in immunoreactivity of atrial natriuretic peptide resulting from neprilysin inhibition. In hypertensive double transgenic rats, LCZ696 caused a dose-dependent and sustained reduction in mean arterial pressure. A healthy participants, a randomized, double-blind, placebo-controlled study confirmed that LCZ696 provided concurrent neprilysin inhibition and AT1 receptor blockade. LCZ696 was safe and well tolerated in human. [2] [3]
References:
McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014 Sep 11;371(11):993-1004.
Gu J, Noe A, Chandra P, Al-Fayoumi S et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010 Apr;50(4):401-14.
Langenickel TH, Dole WP. Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure, Drug Discov Today: Ther Strategies (2014),
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 956.99 |
| Cas No. | 936623-90-4 |
| Formula | C48H54N6Na3O8·2.5H2O |
| Solubility | ≥45.05 mg/mL in DMSO; ≥11.28 mg/mL in H2O; ≥28.5 mg/mL in EtOH |
| SDF | Download SDF |
| Canonical SMILES | O=C(CCC(O[Na])=O)N([C@H](CC(C=C1)=CC=C1C2=CC=CC=C2)C[C@@H](C)C(OCC)=O)[H].O=C(O[Na])[C@@H](N(C(CCCC)=O)CC3=CC=C(C4=CC=CC=C4C5=NN=NN5[Na])C=C3)C(C)C.O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure