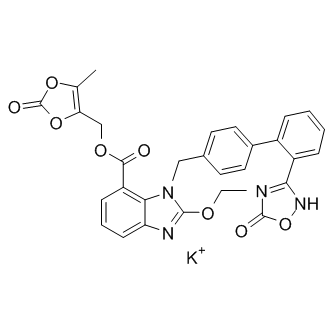
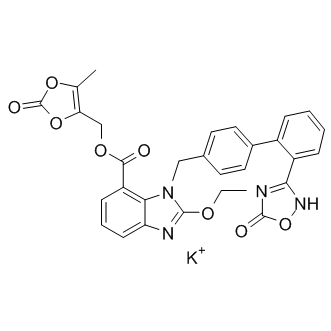
Azilsartan medoxomil monopotassium
Azilsartan medoxomil monopotassium (CAS No. 863031-24-7) is the potassium salt form of Azilsartan medoxomil (CAS No. 863031-21-4, Cat. No.: B2218). Azilsartan medoxomil is a potent and selective angiotensin II type 1 receptor (AT? receptor) antagonist. Its core biological activity is to block the vasoconstriction, aldosterone release, and other effects of angiotensin II by competitively binding to the AT? receptor (with a selectivity of 10,000:1 over the AT? receptor), thereby strongly lowering blood pressure while also having potential renal and cardiovascular protective effects. The IC?? values are well defined: 2.6 nM in radioligand binding assays without drug washout, and still 7.4 nM after 5 hours of washout, with receptor binding affinity significantly higher than other ARB drugs. In vitro receptor binding and functional assays mostly use nM-level concentrations (0.1–100 nM range designed around the IC??). In preclinical animal studies, doses in rat and dog models cover 1–10 mg/kg/day. The effective therapeutic concentrations correspond to clinical doses: adults take 40 mg or 80 mg orally once daily, with 80 mg providing the optimal antihypertensive effect (SUCRA ranking shows a 93% probability of being the best antihypertensive drug), and 24-hour ambulatory systolic/diastolic blood pressure reductions reaching -14.4 mmHg / -7.47 mmHg. The bioavailability is about 60%, the half-life is 11 hours, and the time to peak concentration is 1.5–3 hours. It has good safety, with adverse reactions comparable to other ARB drugs, and is suitable for mild to moderate hypertension, with particularly good tolerability in patients with concomitant diabetes or kidney disease.
References:
[1] Hjermitslev M, Grimm DG, Wehland M, Simonsen U, Krüger M. Azilsartan Medoxomil, an Angiotensin II Receptor Antagonist for the Treatment of Hypertension. Basic Clin Pharmacol Toxicol. 2017 Oct;121(4):225-233. doi: 10.1111/bcpt.12800. Epub 2017 Jun 19. PMID: 28444983.
[2] Zhu L, Wei GC, Xiao Q, Chen QL, Zhao Q, Li XX, Pan LA, Xiong X. Efficacy and safety of azilsartan medoxomil in the treatment of hypertension: a systematic review and meta-analysis. Front Cardiovasc Med. 2024 Jul 4;11:1383217. doi: 10.3389/fcvm.2024.1383217. PMID: 39026999; PMCID: PMC11254823.
[3] Qian J, Zhang M, Chen Z. A Systematic Literature Review and Network Meta-analysis of Azilsartan Medoxomil Compared to Other Anti-hypertensives Efficacy in Lowering Blood Pressure Amongst Mild to Moderate Hypertensive Patients. Adv Ther. 2024 Dec;41(12):4498-4517. doi: 10.1007/s12325-024-02997-5. Epub 2024 Oct 16. PMID: 39412629; PMCID: PMC11550241.
| Storage | Store at -20°C |
| M.Wt | 606.62 |
| Cas No. | 863031-24-7 |
| Formula | C30H23KN4O8 |
| Synonyms | Azilsartan kamedoxomil; TAK 491 monopotassium |
| Solubility | Soluble in DMSO |
| Chemical Name | (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-ethoxy-1-((2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-benzo[d]imidazole-7-carboxylate, potassium salt |
| SDF | Download SDF |
| Canonical SMILES | CCOC1=NC2=CC=CC(=C2N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NOC(=O)[N-]5)C(=O)OCC6=C(OC(=O)O6)C.[K+] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure