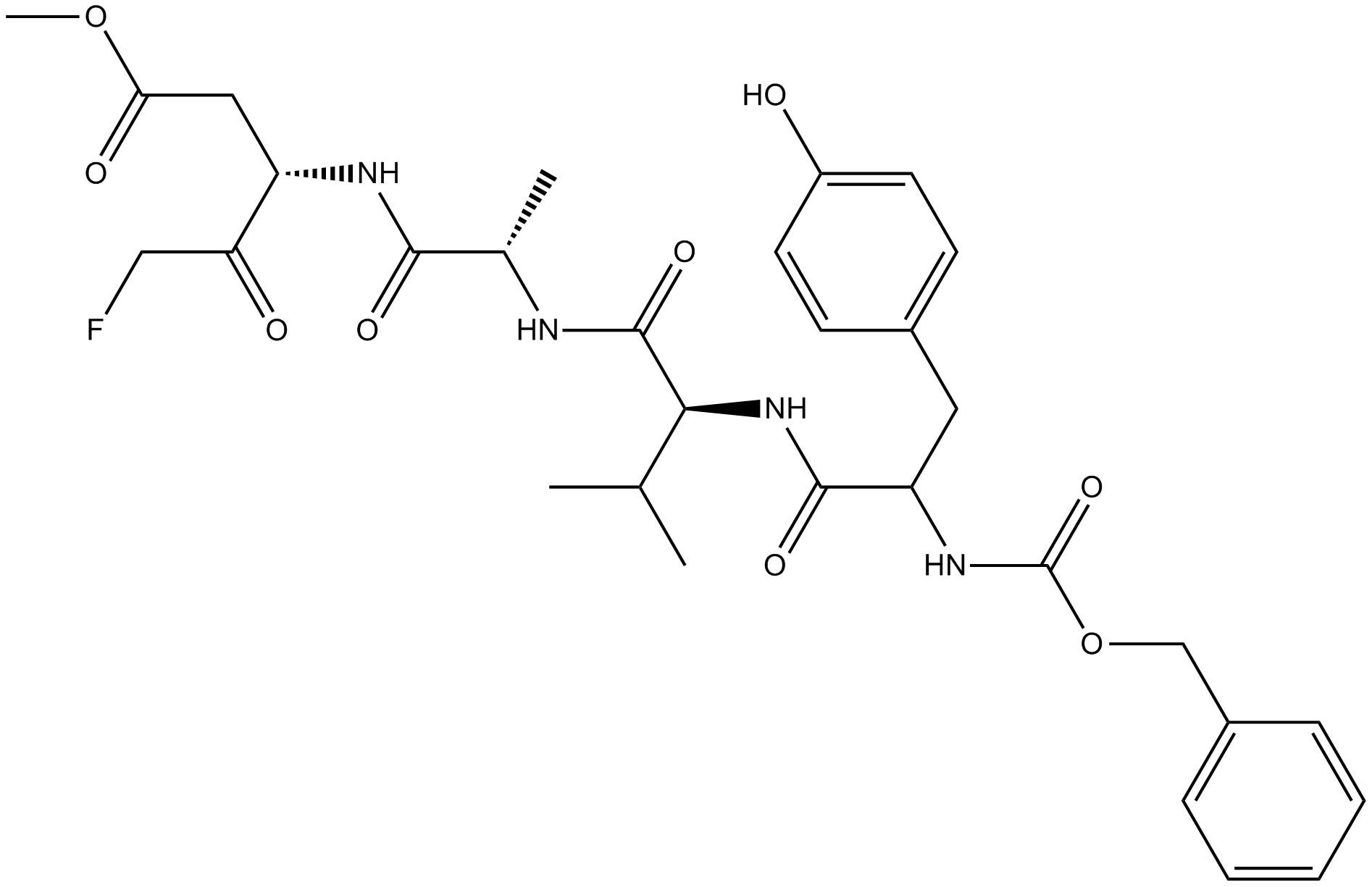
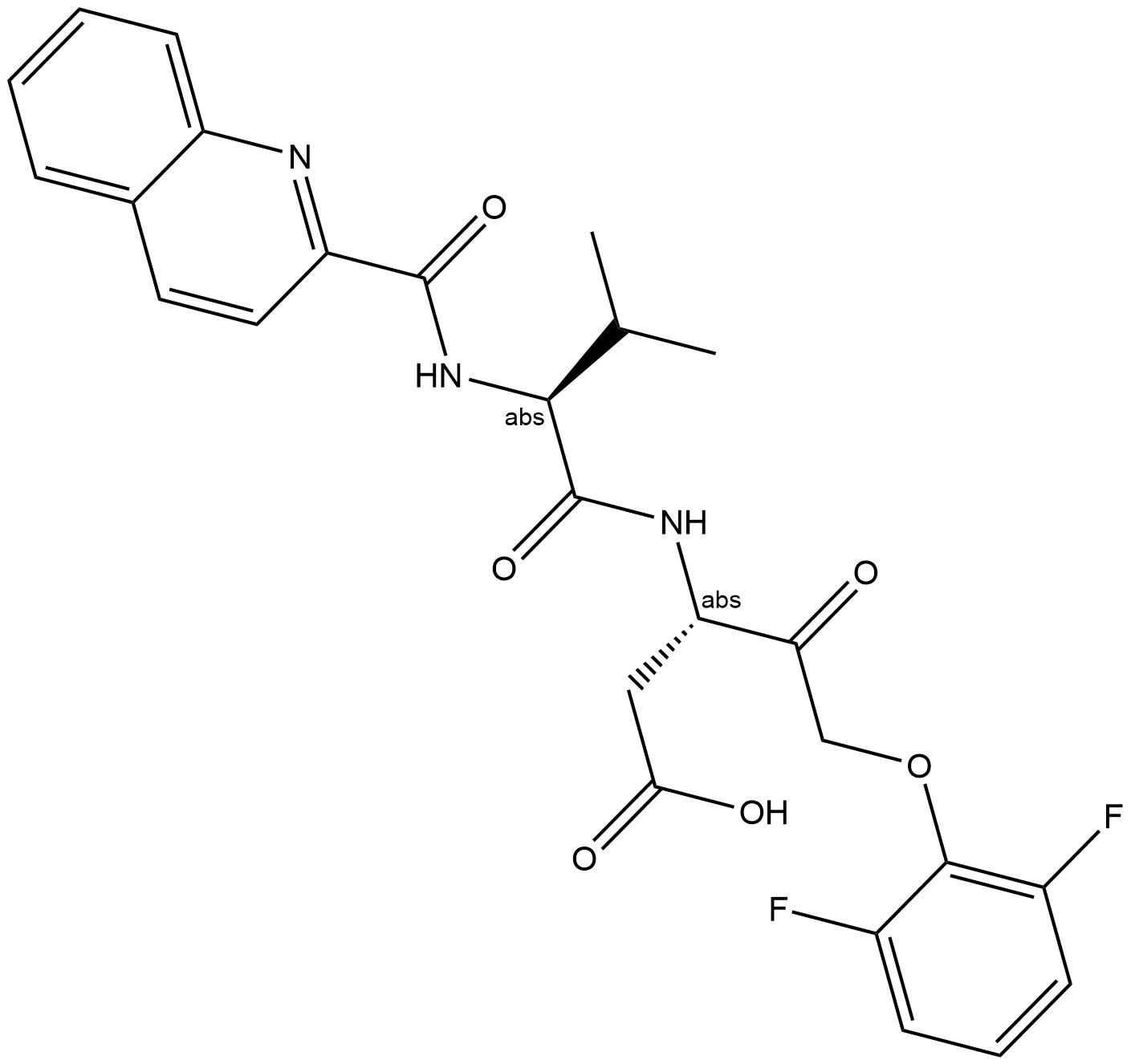
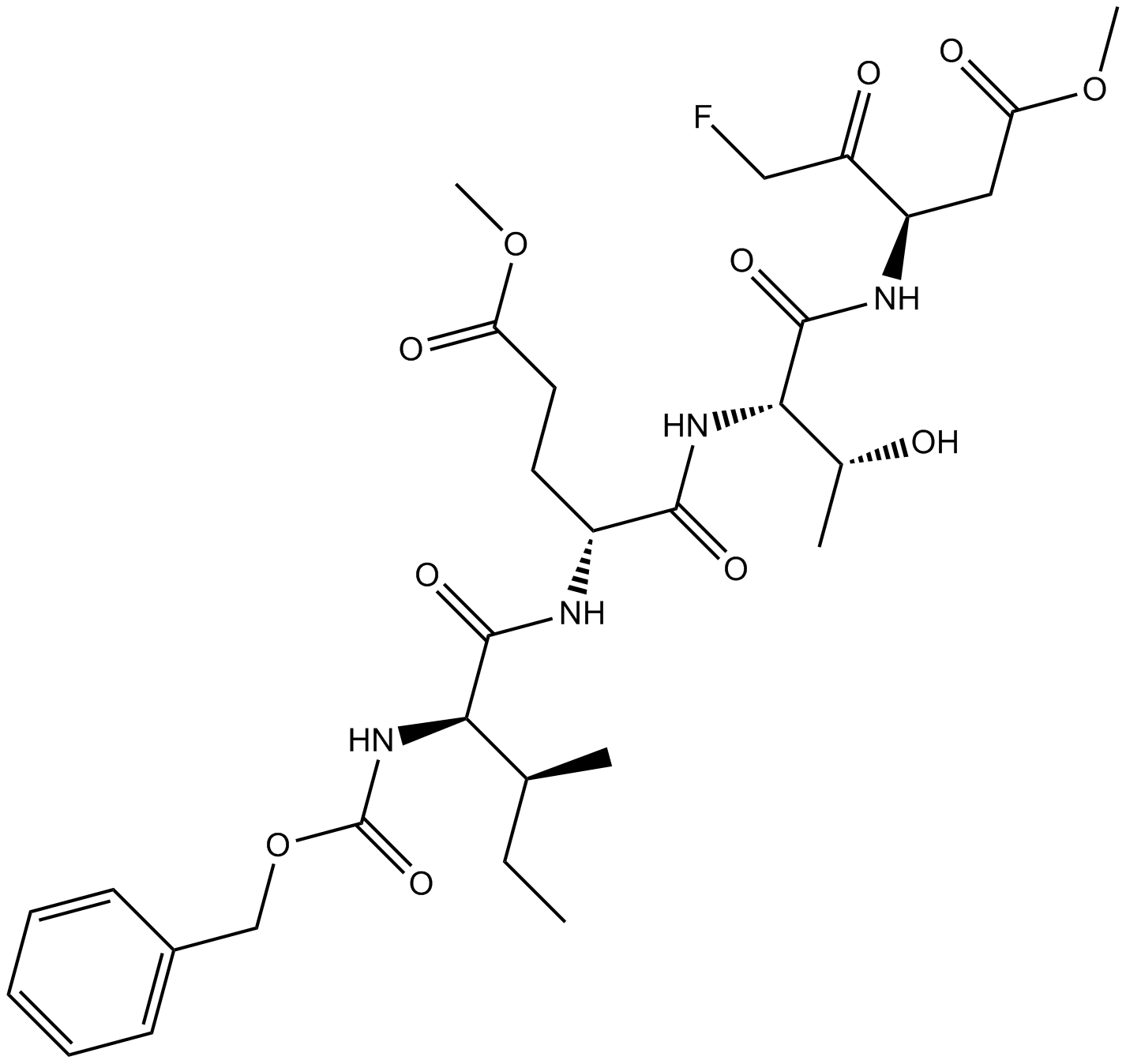
Z-WEHD-FMK
Z-WEHD-FMK (CAS 210345-00-9) is a peptide-based inhibitor primarily targeting inflammatory caspases, including caspase-1, -4, and -5. It irreversibly blocks caspase-mediated proteolytic cleavage, interfering with cellular signaling pathways involved in inflammation and apoptosis. Studies demonstrate that Z-WEHD-FMK treatment prevents Chlamydia-induced fragmentation of the Golgi apparatus by inhibiting golgin-84 cleavage, thereby decreasing bacterial proliferation and altering lipid trafficking to pathogen-containing inclusions. This inhibitor is frequently utilized in cell biology and infectious disease research to investigate caspase-related cellular processes and microbial pathogenesis mechanisms.
- 1. Ravi Padia, Lei Sun, et al. "HOXC8 impacts lung tumorigenesis by preventing pyroptotic cell death through the suppression of caspase-1 expression." Cell Death Dis. 2025 Jul 23;16(1):552 PMID: 40701951
- 2. Huangen Li, Yi Gao, et al. "Tetramethylpyrazine alleviates acute pancreatitis inflammation by inhibiting pyroptosis via the NRF2 pathway." Front Pharmacol. 2025 Apr 23:16:1557681 PMID: 40337514
- 3. Yan-Yan Heng, Hui-Juan Shang, et al. "Sodium tanshinone IIA sulfonate ameliorates neointima by protecting endothelial progenitor cells in diabetic mice." BMC Cardiovasc Disord. 2023 Sep 11;23(1):446. PMID: 37697234
- 4. Shouwen Chen, Peng Jin, et al. "Dual function of a turbot inflammatory caspase in mediating both canonical and non-canonical inflammasome activation." Dev Comp Immunol. 2021 Aug;121:104078. PMID:33794278
- 5. WeiWei, Xiao-XueLi, et al. "Inhibition of vascular neointima hyperplasia by FGF21 associated with FGFR1/Syk/NLRP3 inflammasome pathway in diabetic mice." Atherosclerosis. Available online 29 August 2019.
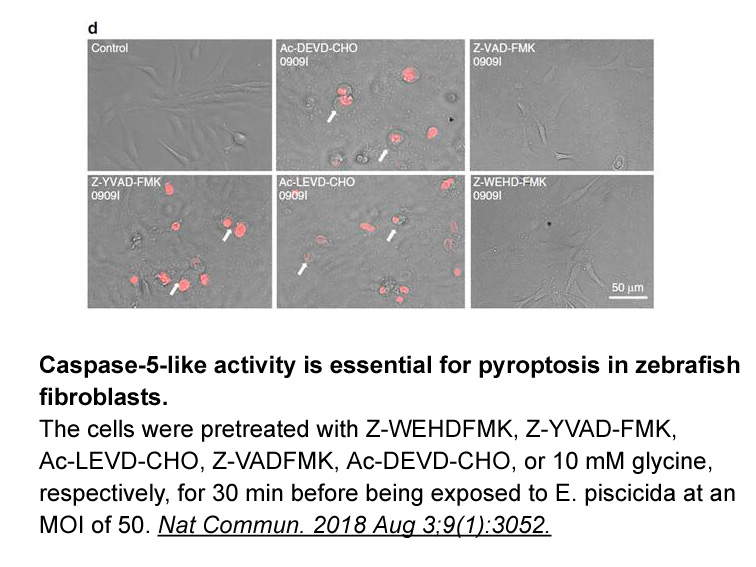
- 6. Yang D, Zheng X, et al. "Sensing of cytosolic LPS through caspy2 pyrin domain mediates noncanonical inflammasome activation in zebrafish." Nat Commun. 2018 Aug 3;9(1):3052. PMID:30076291
| Storage | Store at -20°C |
| M.Wt | 763.77 |
| Cas No. | 210345-00-9 |
| Formula | C37H42FN7O10 |
| Synonyms | Z-Trp-Glu(OMe)-His-Asp(OMe)-FMK |
| Solubility | insoluble in H2O; ≥26.32 mg/mL in EtOH with ultrasonic; ≥46.33 mg/mL in DMSO |
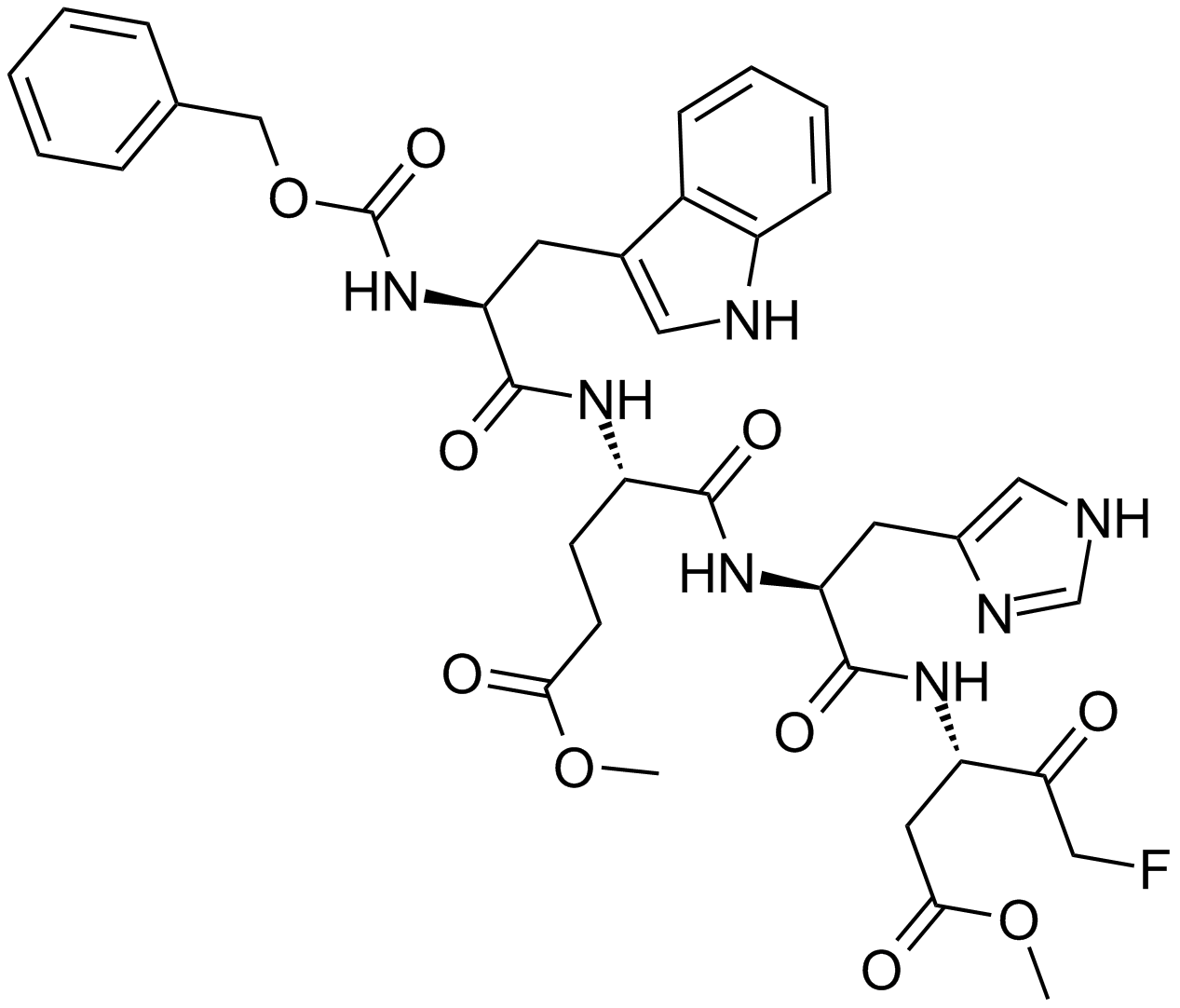
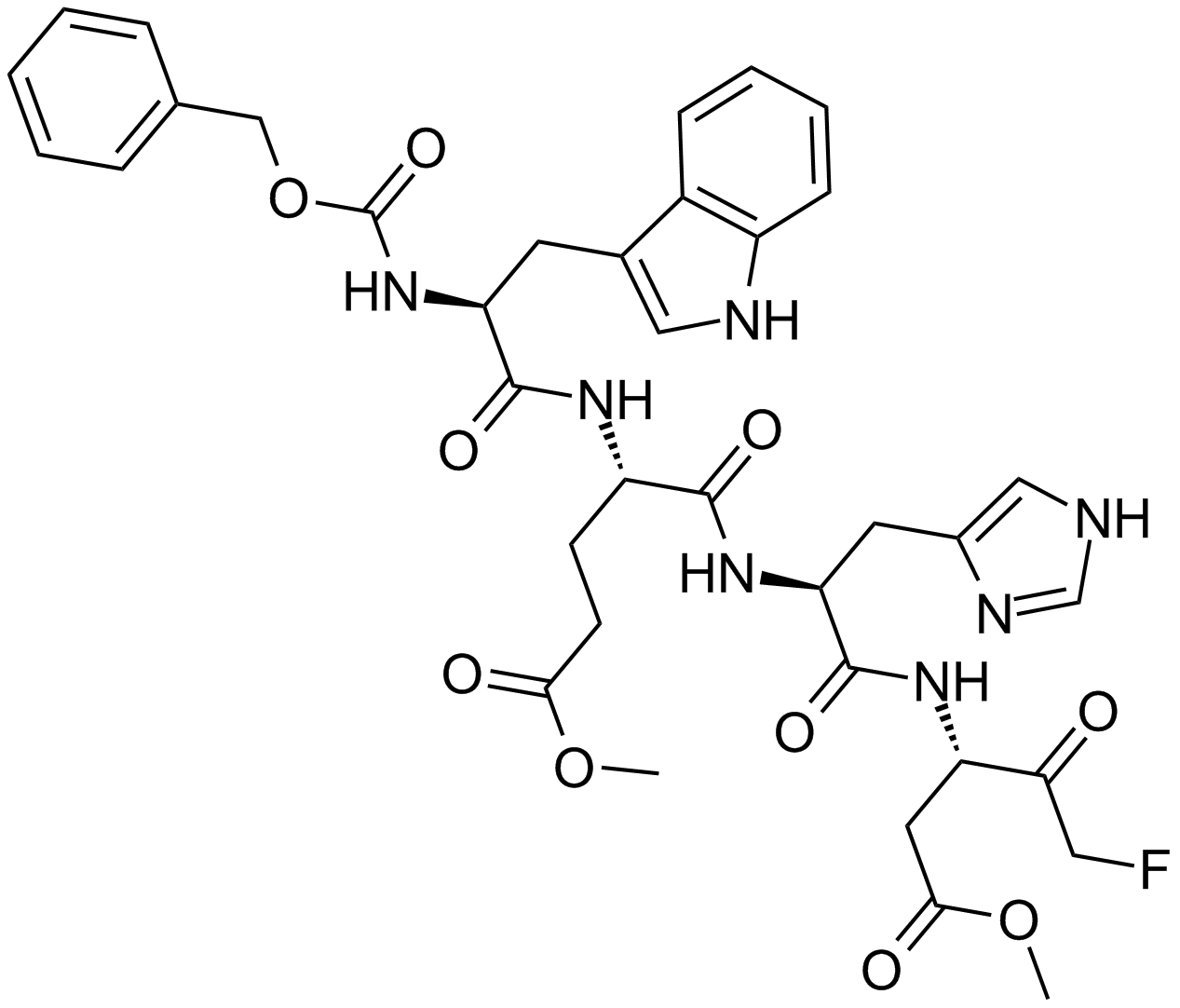
| Chemical Name | methyl (4S)-5-[[(2S)-1-[[(3S)-5-fluoro-1-methoxy-1,4-dioxopentan-3-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-4-[[(2S)-3-(1H-indol-3-yl)-2-(phenylmethoxycarbonylamino)propanoyl]amino]-5-oxopentanoate |
| SDF | Download SDF |
| Canonical SMILES | COC(=O)CCC(C(=O)NC(CC1=CN=CN1)C(=O)NC(CC(=O)OC)C(=O)CF)NC(=O)C(CC2=CNC3=CC=CC=C32)NC(=O)OCC4=CC=CC=C4 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[1] | |
|
Cell lines |
Chlamydia trachomatis (C. trachomatis)-infected HeLa cells |
|
Reaction Conditions |
80 μM Z-WEHD-FMK for 9 h incubation |
|
Applications |
Z-WEHD-FMK (80 μM; 9 h) elicited a near-complete blockage of C. trachomatis-induced cleavage of golgin-84 and increased GM130 expression in cells. Z-WEHD-FMK treatment resulted in a 2-log reduction in numbers of infectious bacteria, which could be reversed by knocking down golgin-84. Thus, Z-WEHD-FMK could effectively prevent Golgi fragmentation, and was used to explore the role of fragmentation of the Golgi compartment in Chlamydia reproduction. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Heuer D, Rejman Lipinski A, Machuy N, et al. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature, 2009, 457(7230): 731-735. |
|
| A synthetic peptide that irreversibly inhibits caspase-5 and related caspase activity. | ||||||
| Targets | caspase-5 | |||||
| IC50 | ||||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data