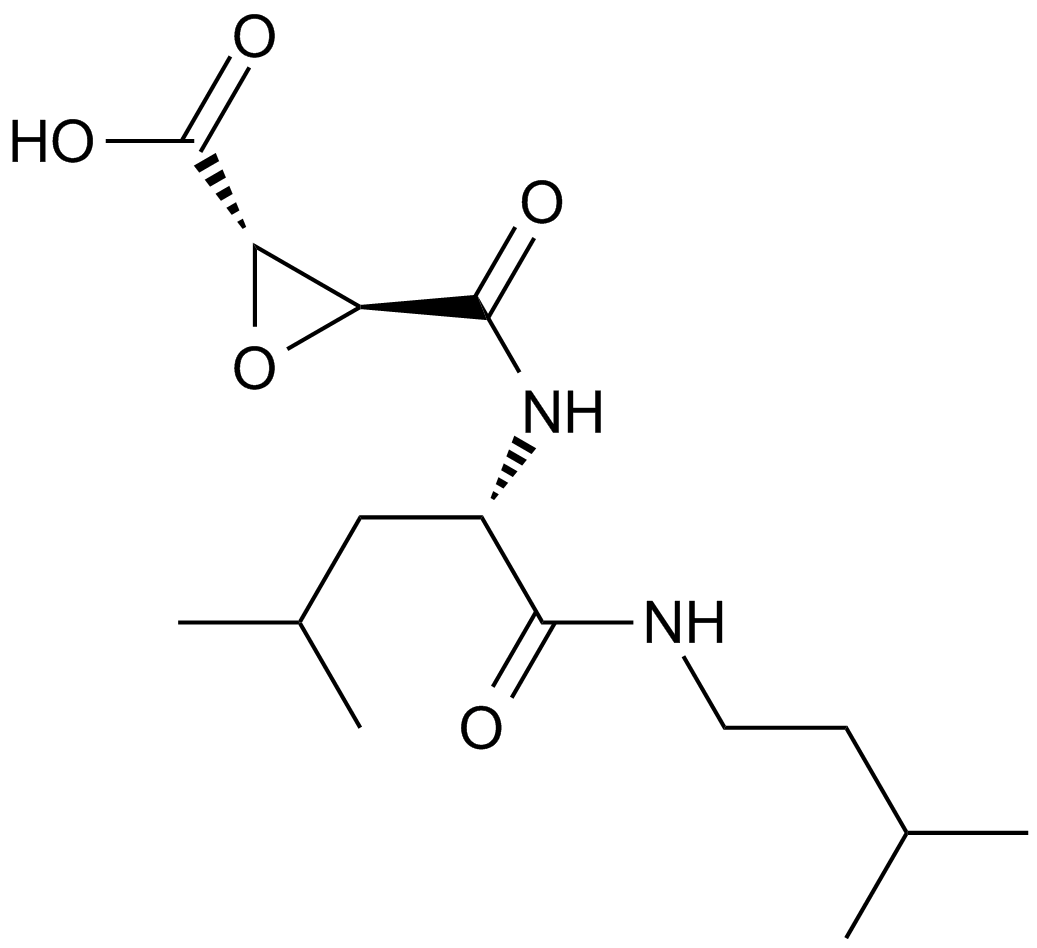
E-64
E-64 (CAS 66701-25-5) is a selective inhibitor targeting cysteine proteases, isolated originally from Aspergillus cultures. Structurally classified as an L-trans-epoxysuccinyl peptide, it irreversibly inhibits proteases such as papain, ficin, and bromelain by covalent binding to the active-site cysteine residue. Its inhibitory activity additionally extends to mammalian cysteine proteases including cathepsins B, H, and L as well as calcium-dependent protease calpain, with reported IC50 values ranging approximately between 10-100 nM depending on specific enzyme assays and conditions. In research laboratories, E-64 is primarily utilized for mechanistic studies, active-site titration assays, and quantitative evaluation of cysteine protease concentrations and enzyme kinetics.
References:
1. A. J. BARRETT, A. A. KEMBHAVI, L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem. J. (1982) 201, 189-198
2. Hanada, K., Tamai, M., Yamagishi, M., Ohmura, S., Sawada, J. & Tanaka, I. (1978c) Agric. Biol. Chem. 42, 523-528
3. Towatari, T., Tanaka, K., Yoshikawa, D. & Katunuma, N. (1978).J. Biochem. (Tokyo) 84, 659-671.
4. Mort, J. S., Recklies, A. D. & Poole, A. R. (1980) Biochim. Biophys. Acta 614, 134-143.
5. Sugita, H., Ishiura, S., Suzuki, K. & Imahori, K. (1980) J. Biochem. (Tokyo) 87, 339-341
6. Hanada, K., Tamai, M., Morimoto, S., Adachi, T.,Ohmura, S., Sawada, J. & Tanaka, I. (1978a) Agric. Biol. Chem. 42, 537-541.
7. Hanada, K., Tamai, M., Ohmura, S., Sawada, J., Seki, T.& Tanaka, I. (1978b)Agric. Biol. Chem. 42, 529-536
8. Knight, C. G. (1980) Biochem. J. 189,447-453
9. Takio, K., Towatari, T., Katunuma, N. & Titani, K.(1980) Biochem. Biophys. Res. Commun. 97, 340-346
10. Bender, M. L., Begue-Canton, M. L., Blakeley, R. L.,Brubacher, L. J., Feder, J., Gunter, C. R., Kezdy, F. J.,Killheffer, J. V., Marshall, T. H., Miller, C. G., Roeske,R. W. & Stoops, J. K. (1966) J. Am. Chem. Soc. 88,5890-5913
- 1. Andrew Thorne, Akanksha Bansal, et al. "Differential regulation of BIRC2 and BIRC3 expression by inflammatory cytokines and glucocorticoids in pulmonary epithelial cells." PLoS One. 2023 Jun 8;18(6):e0286783. PMID: 37289679
- 2. Cliff J. Luke, Stephanie Markovina, et al. "Lysoptosis is an evolutionarily conserved cell death pathway moderated by intracellular serpins." Commun Biol. 2022 Jan 12;5(1):47. PMID: 35022507
- 3. Zhijun Liu, Himani Nailwal, et al. "A class of viral inducer of degradation of the necroptosis adaptor RIPK3 regulates virus-induced inflammation." Immunity. 2021 Feb 9;54(2):247-258.e7. PMID: 33444549
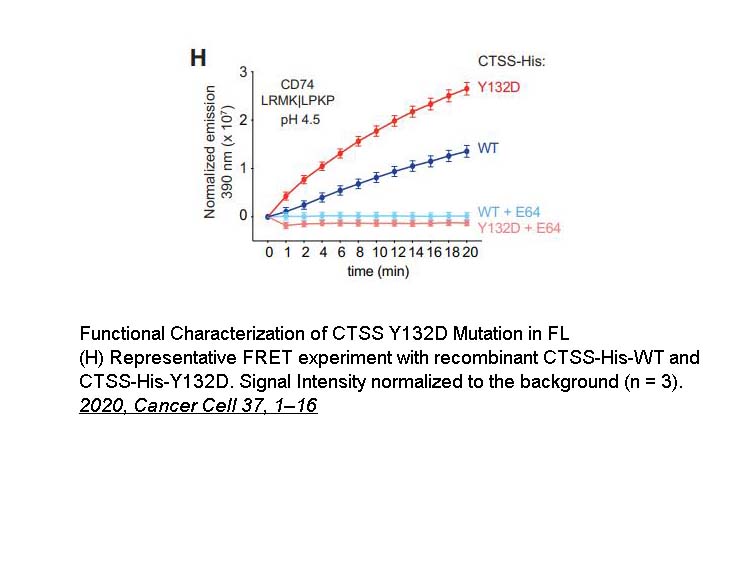
- 4. Dheilly E, Battistello E, et al. "Cathepsin S Regulates Antigen Processing and T Cell Activity in Non-Hodgkin Lymphoma." Cancer Cell. 2020;37(5):674-689.e12. PMID: 32330455
- 5. Blass G, Levchenko V, et al. "Chronic cathepsin inhibition by E-64 in Dahl salt-sensitive rats." Physiol Rep. 2016 Sep;4(17). pii: e12950. PMID: 27597769
- 6. Ying Long, Xuri Zhang, et al. "Initial events in the breakthrough of the epithelial barrier of the small intestine by Angiostrongylus cantonensis." Arch Biol Sci. 2016;68(2):375-383
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 357.41 |
| Cas No. | 66701-25-5 |
| Formula | C15H27N5O5 |
| Solubility | ≥49.1 mg/mL in H2O; ≥53.6 mg/mL in DMSO; ≥55.2 mg/mL in EtOH |
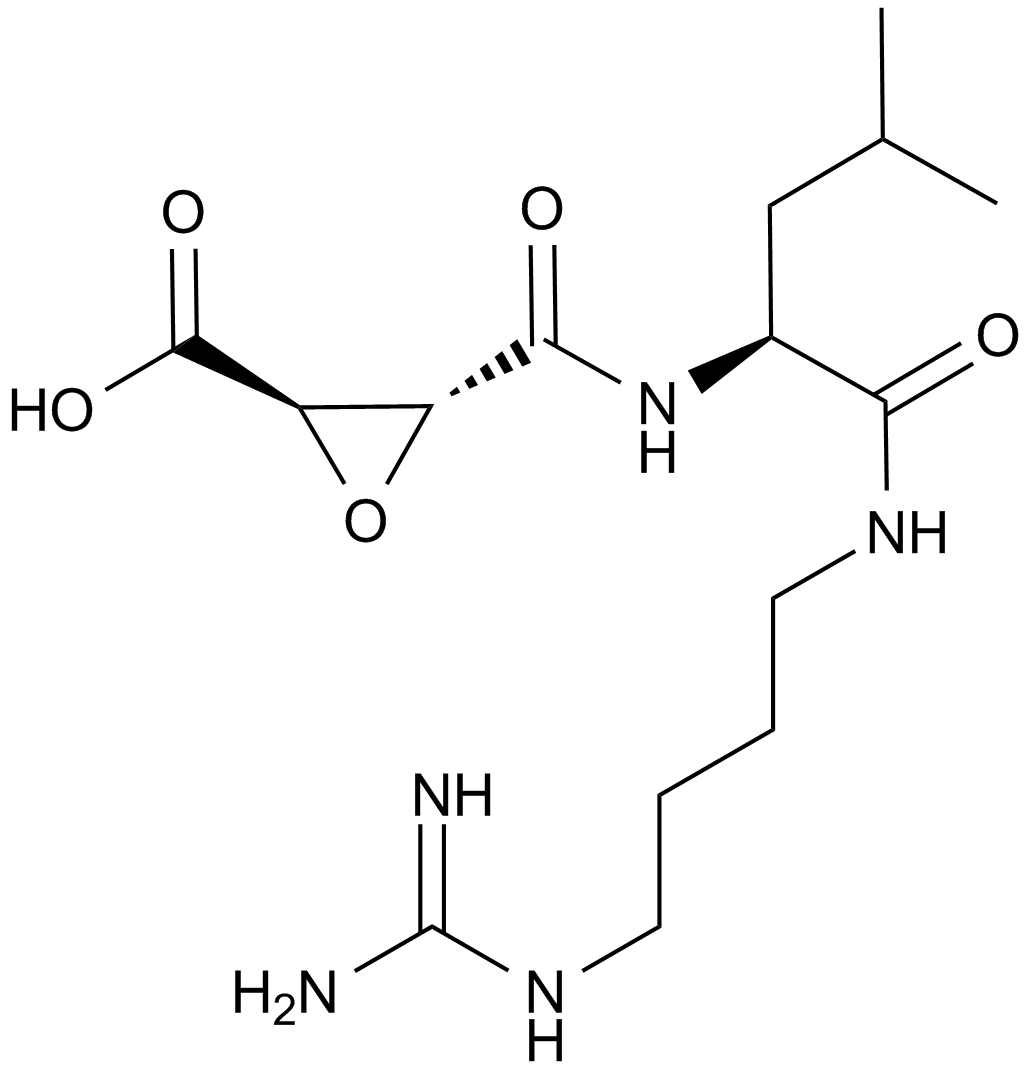
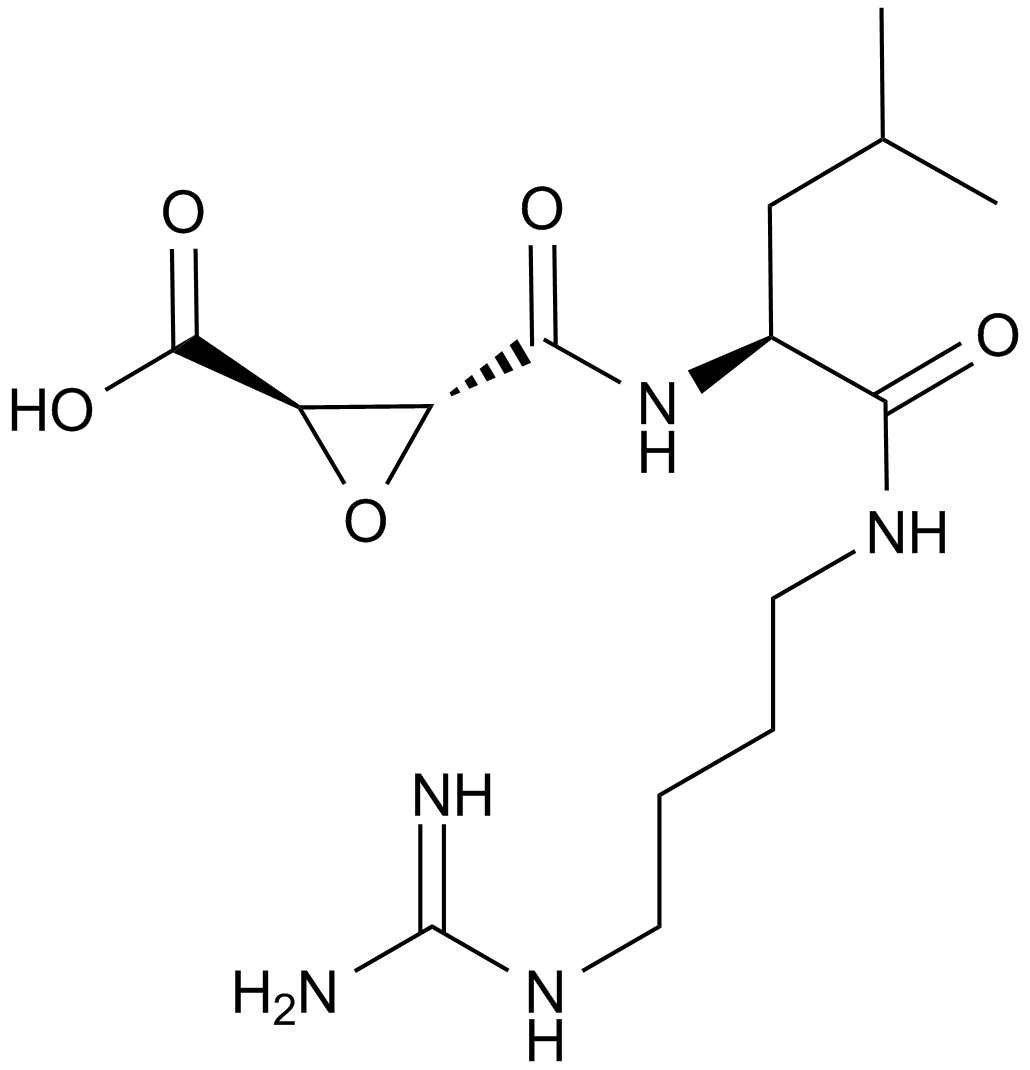
| Chemical Name | (2S,3S)-3-[[(2S)-1-[4-(diaminomethylideneamino)butylamino]-4-methyl-1-oxopentan-2-yl]carbamoyl]oxirane-2-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | CC(C)C[C@@H](C(NCCCCN=C(N)N)=O)NC([C@H]1O[C@@H]1C(O)=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment: [1] | |
|
Cell lines |
H-59 and M-27 cells |
|
Preparation method |
The solubility of this compound in DMSO is ≥53.6 mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
10 μg/mL, 48 hours |
|
Applications |
E-64 inhibited H-59 invasion in a dose-dependent manner with a maximal inhibition of 97% at a concentration of 10 μg/mLwhich was non-toxic. Cell migration as measured with filters coated with 7.5 μg/filter type IV collagen was reduced by only 25% suggesting that the cysteine proteinases played a more minor role in cell migration in the absence of a basement membrane barrier. On the other hand M-27 invasion was not significantly affected by treatment with E-64 even at concentrations as high as 100 μg/mL. |
| Animal experiment: [2] | |
|
Animal models |
Wistar strain rats |
|
Dosage form |
Intraperitoneal injection, 1 mg/100 g body weight |
|
Applications |
The animals were killed 1 h after the injection and the cathepsin B and cathepsin L activities in the lysosomal were assayed. The inhibition caused by E-64 was already detectable 1 hour after its injection. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Navab R, Mort J S, Brodt P. Inhibition of carcinoma cell invasion and liver metastases formation by the cysteine proteinase inhibitor E-64. Clinical & experimental metastasis, 1997, 15(2): 121-129. [2] Hashida S, TOWATARI T, KOMINAMI E, et al. Inhibitions by E-64 derivatives of rat liver cathepsin B and cathepsin L in vitro and in vivo. Journal of biochemistry, 1980, 88(6): 1805-1811. |
|
| E-64 is a natural, potent, and irreversible inhibitor of cysteine proteases.Its IC50 values for inhibiting cathepsins K, S, and L, in vitro, are 1.4, 4.1, and 2.5 nM, respectively. | ||||||
| Targets | cathepsins K | cathepsins S | cathepsins L | |||
| IC50 | 1.4 nM | 4.1 nM | 2.5 nM | |||
Quality Control & MSDS
- View current batch:
Chemical structure
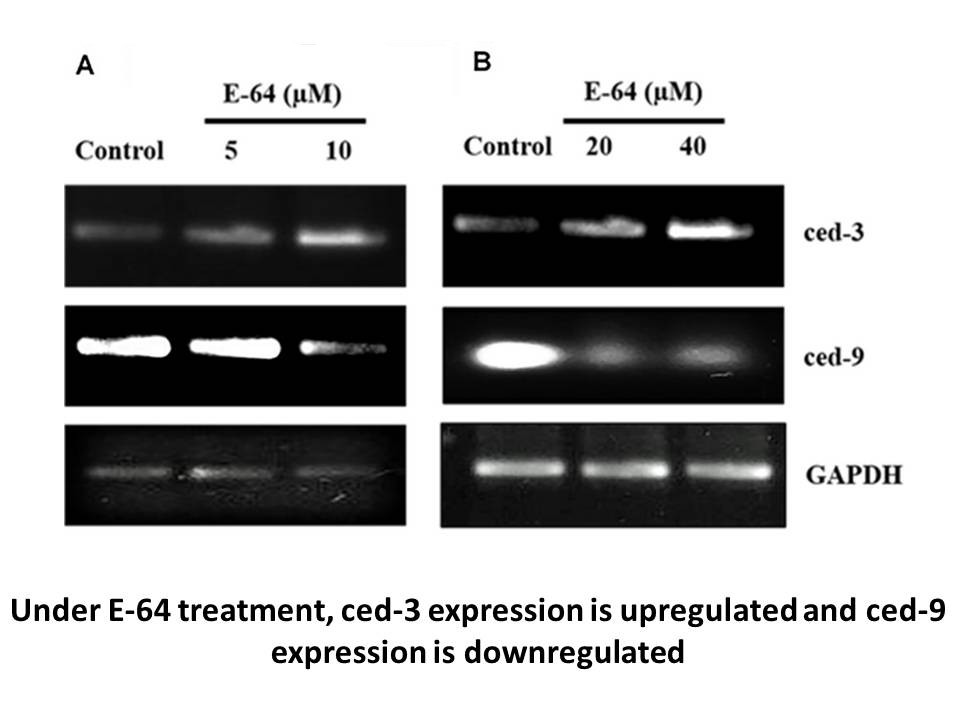
Related Biological Data
Related Biological Data
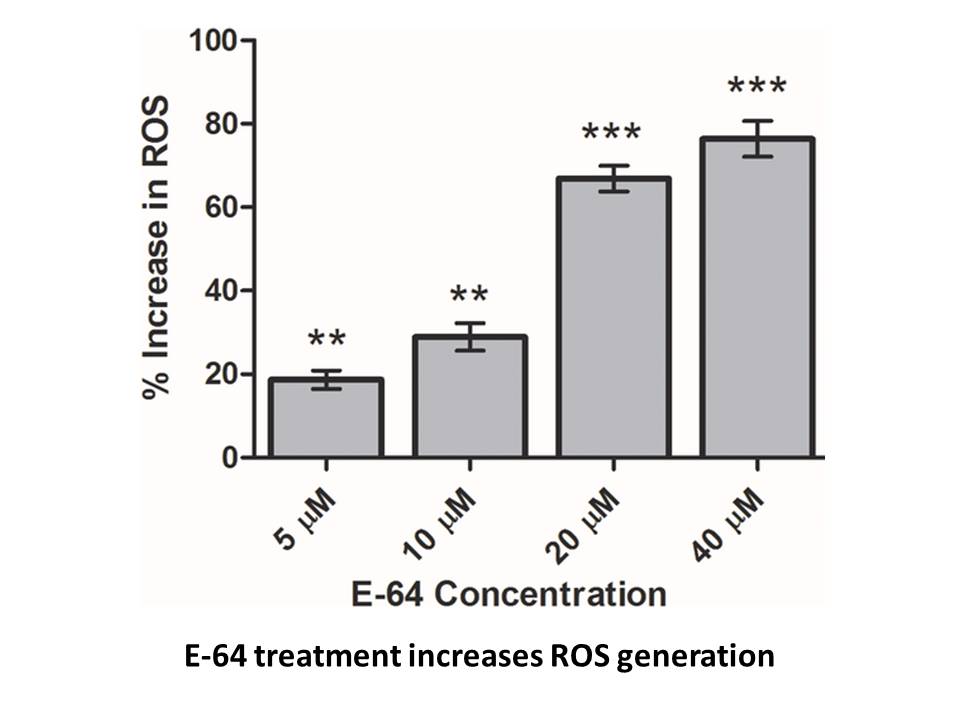
Related Biological Data
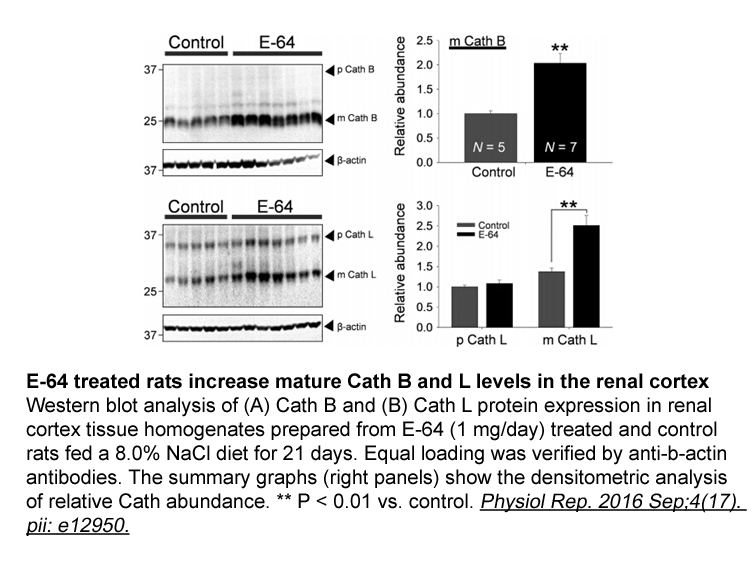
Related Biological Data
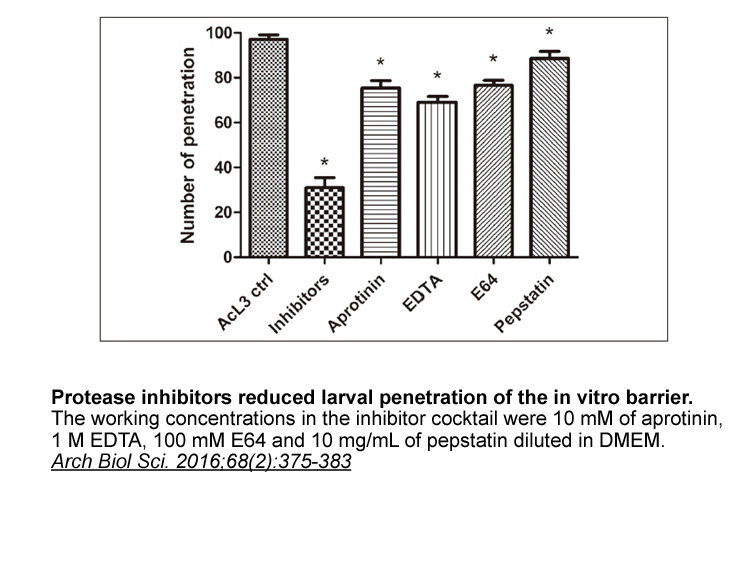
Related Biological Data