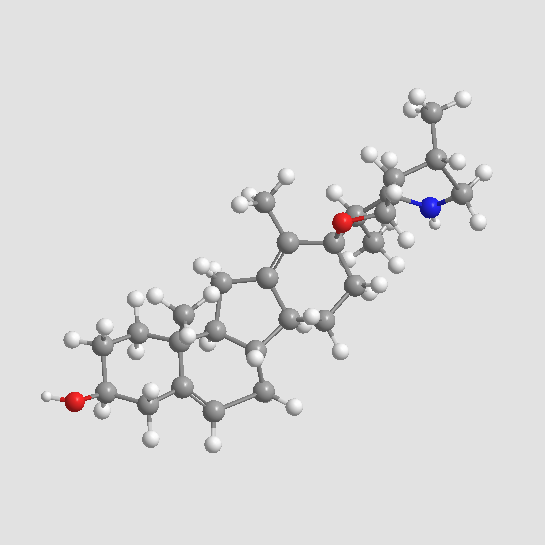
Cyclopamine
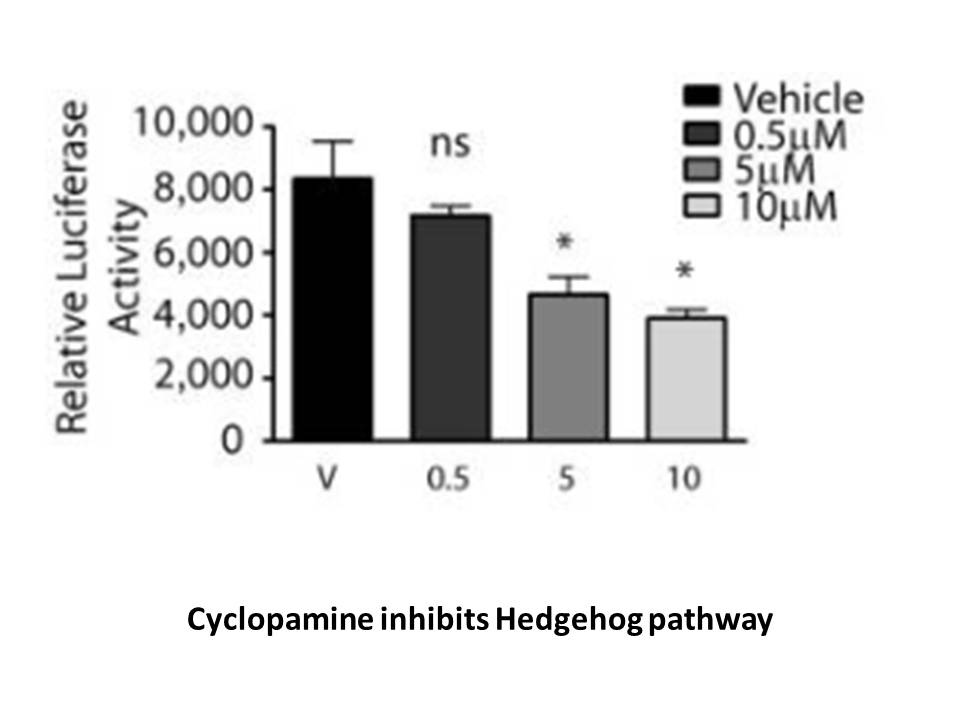
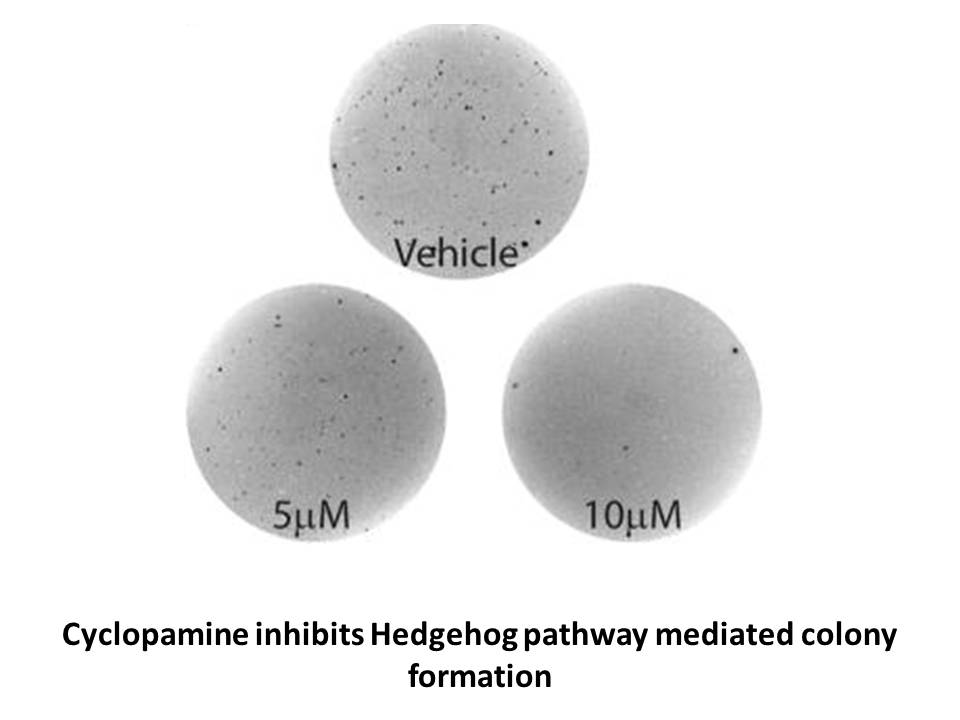
Cyclopamine is a naturally occurring Hedgehog (Hh)?specific small?molecule signaling steroidal alkaloid inhibitor, causes a profound inhibition of tumor growth, has significant anti?invasive, anti?proliferative and anti?estrogenic potency in human breast cancer cells [2] [1]. The EC50 of cyclopamine is 10.57 μM, it was identified by an FXR-bla (farnesoid X receptor- b-lactamase) assay [3].
Hh signaling pathway plays a critical role in embryonic development and tumorigenesis [4]. Hh signaling pathway shows saliency in regulating cellular proliferation and differentiation in a wide array of human tissues. It is related to aberrant cell survival in numerous human malignancies, ranging from BCCs and medulloblastomas to small cell lung, gastrointestinal, breast and prostate tumors [1].
Treated with cyclopamine (10 or 20 μM) only and incubated for time periods ranging from 0 to10 days, MCF-7 cells and MDA?MB?231 cells displayed a significant reduction in proliferation rate compared with the control cells on days 3 and 6 (P
Embryoes exposed to cyclopamine resulted in visible external defects, including cyclopia, proboscis formation, microphthalmia, thoracic lordosis, amelia and decreased body size. Examination of gastrointestinal organs revealed severe deficits, including less length of the gut tube and mesenchymal cell numbers in foregut-derived organs. Ectopic structures in duodenum, stomach, and dorsal pancreas were also found [5].
References:
[1]. Marc J. Meth and Jeffrey M. Weinberg. Cyclopamine: Inhibiting Hedgehog in the Treatment of Psoriasis. Continuing Medical Education, 2006, 78: 185-188.
[2]. Jun Che, Fu-Zheng Zhang, Chao-Qian Zhao, et al. Cyclopamine is a novel Hedgehog signaling inhibitor with significant anti?proliferative, anti?invasive and anti?estrogenic potency in human breast cancer cells. Oncology Letters, 2013, 5: 1417-1421.
[3]. Chia-Wen Hsu, Jinghua Zhao, Ruili Huang, et al. Quantitative High-Throughput Profiling of Environmental Chemicals and Drugs that Modulate Farnesoid X Receptor. Scientific Reports, 2014, 4: 6437.
[4]. Robert J. Lipinski, Paul R. Hutson, Paul W. Hannam, et al. Dose- and Route-Dependent Teratogenicity, Toxicity, and Pharmacokinetic Profiles of the Hedgehog Signaling Antagonist Cyclopamine in the Mouse. Toxicological Sciences, 2008, 104(1):189-197.
[5]. Seung K. Kim and Douglas A. Melton. Pancreas development is promoted by cyclopamine, a Hedgehog signaling inhibitor. Proc. Natl. Acad. Sci. USA, 1998, 95: 13036-13041.
- 1. Houbo Jiang, Zichun Xiao, et al. "Generation of human induced pluripotent stem cell-derived cortical neurons expressing the six tau isoforms." J Alzheimers Dis. 2025 Apr 23:13872877251334831. PMID: 40267294
- 2. Shanshan Wang, Zhengui Zheng. "Differences in Formation of Prepuce and Urethral Groove During Penile Development Between Guinea Pigs and Mice Are Controlled by Differential Expression of Shh, Fgf10 and Fgfr2." Preprints.org 3 January 2025
- 3. Jun Zou, Sha Yang, et al. "miR-630 as a therapeutic target in pancreatic cancer stem cells: modulation of the PRKCI-Hedgehog signaling axis." Biol Direct. 2024 Nov 11;19(1):109 PMID: 39529141
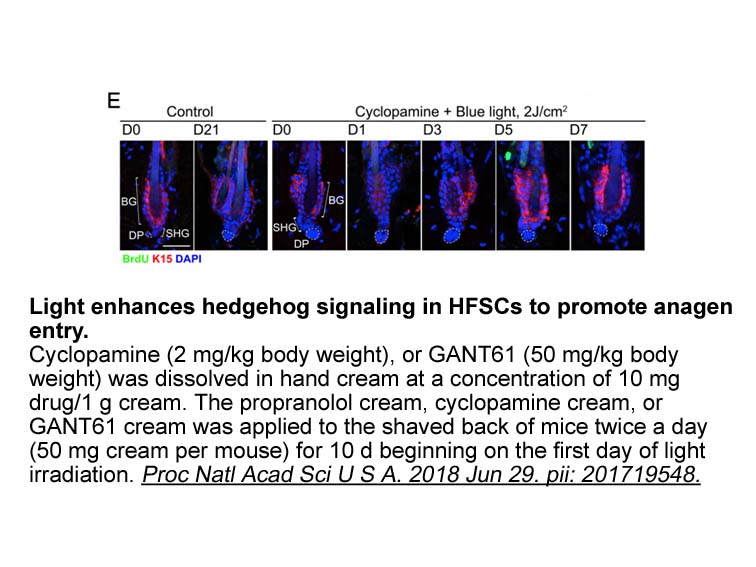
- 4. Fan SM, Chang YT, et al. "External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway." Proc Natl Acad Sci U S A. 2018 Jul 17;115(29):E6880-E6889. PMID:29959210
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 411.62 |
| Cas No. | 4449-51-8 |
| Formula | C27H41NO2 |
| Synonyms | 11-Deoxojervine |
| Solubility | insoluble in EtOH; insoluble in H2O; ≥6.86 mg/mL in DMSO |
| Chemical Name | (3S,3'R,3'aS,6'S,6aS,6bS,7'aR,9R,11aS,11bR)-3',6',10,11b-tetramethylspiro[2,3,4,6,6a,6b,7,8,11,11a-decahydro-1H-benzo[a]fluorene-9,2'-3a,4,5,6,7,7a-hexahydro-3H-furo[3,2-b]pyridine]-3-ol |
| SDF | Download SDF |
| Canonical SMILES | CC1CC2C(C(C3(O2)CCC4C5CC=C6CC(CCC6(C5CC4=C3C)C)O)C)NC1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment: [1] | |
|
Cell lines |
AA/C1, RG/C2, CaCo2, HT29 and SW480 cells |
|
Preparation method |
The solubility of this compound in DMSO is |
|
Reaction Conditions |
20 μM, 48 hours for cell yield inhibition 10 μM, 48 hours for apoptosis induction (measured by PARP expression) |
|
Applications |
Treatment of cyclopamine significantly reduced cell yield in all the tested human colorectal tumour cell lines with a dose-dependent manner. Cyclopamine also remarkably induced apoptosis in each of the cell lines. The CaCo2 cell line showed particular sensitivity to cyclopamine-induced apoptosis. |
| Animal experiment: [2] | |
|
Animal models |
C57BL/6J mice |
|
Dosage form |
Intraperitoneal injection, 160 mg/kg/day for 31 hours. |
|
Applications |
Cyclopamine showed teratogenic potential in the tested animals. Affected embryos were slightly smaller than normal littermates and exhibited mild blunting of the snout as well as cleft lip and palate. Embryos exhibited unilateral and bilateral complete cleft lip with clefts extending into the primary and secondary palate. Facial clefts were often accompanied by open eyelid defects and in one embryo by forelimb syndactyly. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Qualtrough D, Buda A, Gaffield W, et al. Hedgehog signalling in colorectal tumour cells: induction of apoptosis with cyclopamine treatment. International journal of cancer, 2004, 110(6): 831-837. [2] Lipinski R J, Hutson P R, Hannam P W, et al. Dose-and route-dependent teratogenicity, toxicity, and pharmacokinetic profiles of the hedgehog signaling antagonist cyclopamine in the mouse. Toxicological sciences, 2008, 104(1): 189-197. |
|
| Description | Purmorphamine is a blocker of BODIPY-cyclopamine binding to Smo with IC50 of ~ 1.5 μM and also is an inducer of osteoblast differentiation with EC50 of 1 μM. | |||||
| Targets | Smoothened (Smo) | |||||
| IC50 | 46 nM | |||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data