BMS-833923
BMS-833923 is an orally bioavailable and selective antagonist of smoothened (SMO) with IC50 value of 5.8 nM in NIH3T3 cell line [1].
The Hedgehog (Hh) signaling is a critical pathway involved in embryonic development and in tissue maintenance and repair in adults. It consists of the Hh ligands, the transmembrane receptors Patched 1 and 2, the G-protein- coupled receptor-like protein SMO and the glioma-associated oncogene transcription factors GLI1 to 3. The aberrant activation of Hh pathway, both mutational and epigenetic, is found to be associated with multiple aspects of tumorigenesis in various tumor cells. As a smoothened inhibitor, BMS-833923 can block the binding of cyclopamine (a naturally occurring SMO inhibitor) to SMO. It showed potent Hh pathway inhibitory activity with IC50 values at nanomolar in multiple cell-based assays. BMS-833923 also potently inhibited Hh pathway in medulloblastoma and pancreatic carcinoma xenograft models [1, 2 and 3].
In vitro, BMS-833923 inhibited the expression of GLI1 and PTCH1 in cell lines expressing wild-type SMO or activated mutant SMO with IC50 values in the range from 6 to 35 nM. In the FACS-based binding assays, it does-dependently suppressed cyclopamine binding to SMO with IC50 value of 21 nM. In the esophageal adenocarcinoma cell lines OE19 and OE33, treatment of BMS-833923 significantly reduced cell proliferation with IC50 values of both 10 μM. Besides that, BMS-833923 was found to inhibit the growth of multiple myeloma cells and the proportion of ALDH+ cancer stem cells. It also inhibited the growth of many other tumor cells derived from patients with hematological malignancies including ALL, AML and CML [3 and 4].
In animal models with medulloblastoma and pancreatic carcinoma xenografts, administration of BMS-833923 at single oral dose showed robust inhibition of Hh pathway. In a rat model with gastroesophageal reflux disease, the administration of BMS-833923 at dose of 10 mg/kg/day resulted in the decreased development of both Barrett esophagus and esophageal adenocarcinoma by 35.7% [3 and 5].
References:
[1] Siu L L, Papadopoulos K P, Alberts S, et al. A first-in-human phase 1study of an oral hedgehog pathway antagonist, BMS-833923 (XL139), in subjects with advanced or metastatic solid tumors. Mol Cancer Ther, 2009, 8(12 Suppl): A55.
[2] Justilien V, Fields A P. Molecular Pathways: Novel Approaches for Improved Therapeutic Targeting of Hedgehog Signaling in Cancer Stem Cells. Clinical Cancer Research, 2015, 21(3): 505-513.
[3] Gendreau S B, Hawkins D, Ho C P, et al. Abstract B192: Preclinical characterization of BMS‐833923 (XL139), a hedgehog (HH) pathway inhibitor in early clinical development. Molecular Cancer Therapeutics, 2009, 8(12 Supplement): B192-B192.
[4] Zaidi A H, Komatsu Y, Kelly L A, et al. Smoothened inhibition leads to decreased proliferation and induces apoptosis in esophageal adenocarcinoma cells. Cancer investigation, 2013, 31(7): 480-489.
[5] Gibson M K, Zaidi A H, Davison J M, et al. Prevention of Barrett esophagus and esophageal adenocarcinoma by smoothened inhibitor in a rat model of gastroesophageal reflux disease. Annals of surgery, 2013, 258(1): 82-88.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 473.57 |
| Cas No. | 1059734-66-5 |
| Formula | C30H27N5O |
| Synonyms | BMS 833923;BMS833923;XL-139;XL139;XL 139 |
| Solubility | ≥47.4 mg/mL in DMSO; insoluble in H2O; ≥5.14 mg/mL in EtOH with gentle warming and ultrasonic |
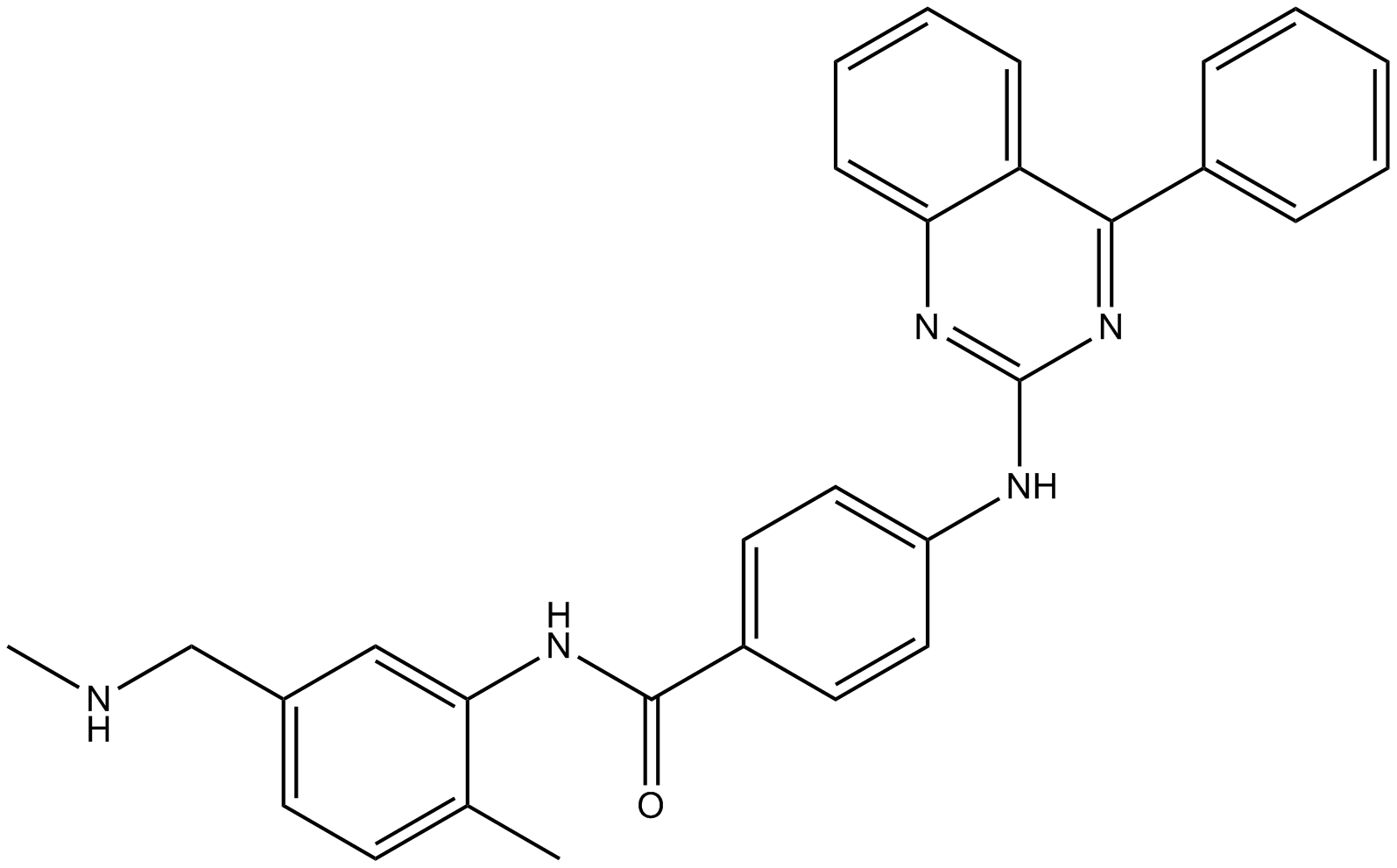
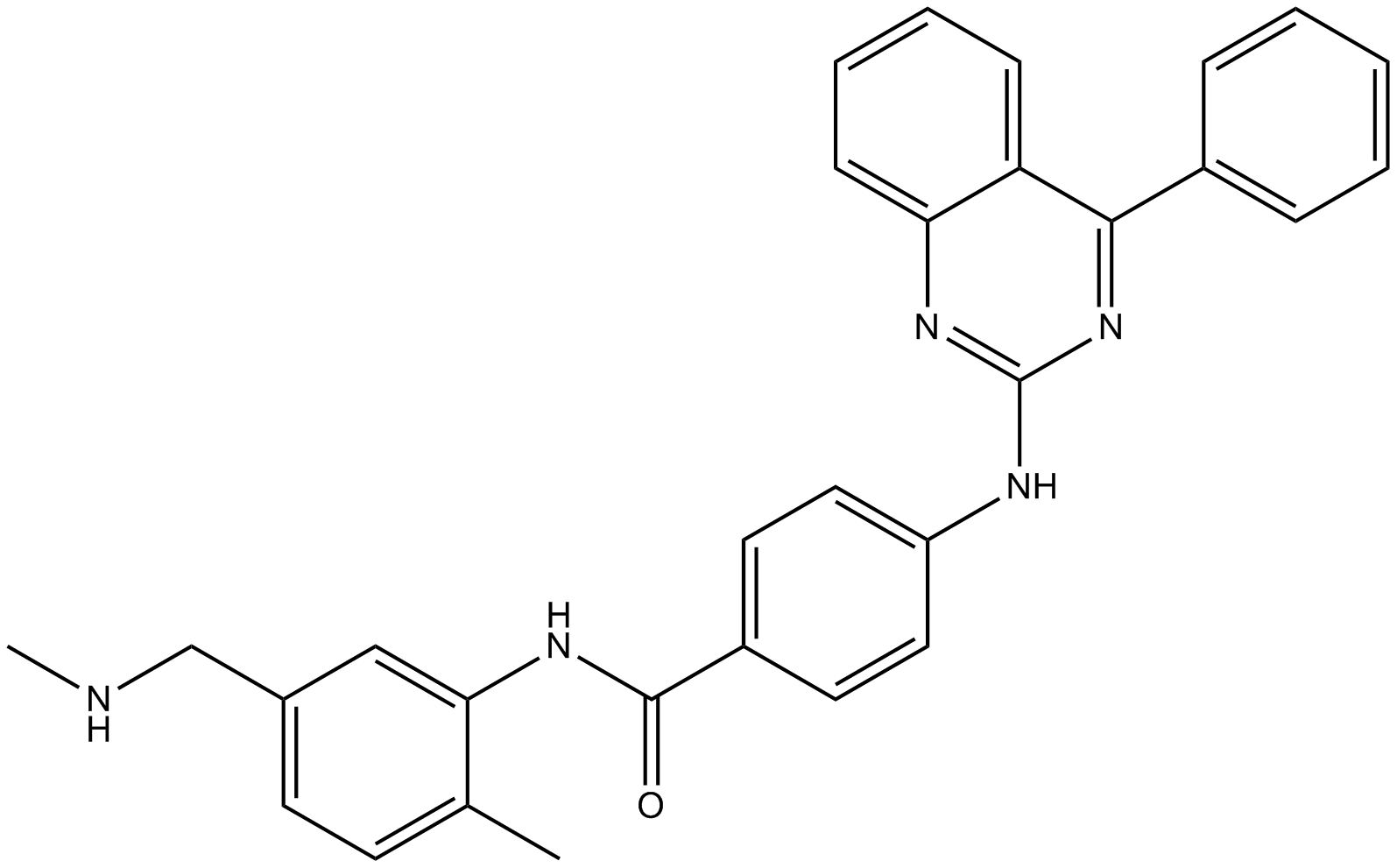
| Chemical Name | N-[2-methyl-5-(methylaminomethyl)phenyl]-4-[(4-phenylquinazolin-2-yl)amino]benzamide |
| SDF | Download SDF |
| Canonical SMILES | Cc(ccc(CNC)c1)c1NC(c(cc1)ccc1Nc1nc(cccc2)c2c(-c2ccccc2)n1)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
OE19 (JROECL19) and OE33 (JROECL33) esophageal adenocarcinoma(EAC) cell lines |
|
Preparation method |
The solubility of this compound in DMSO is >47.4mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0 to 100 μM for 24 and 48 hr |
|
Applications |
In OE19 and OE33 cells, BMS-833923 (10 μM) inhibited cell proliferation with the IC50 of 10 μM. BMS-833923 (25 μM) completely inhibited cell proliferation. In OE19 and OE33 cells, treatment with 10 μM BMS-833923 resulted in 82 and 73.4% apoptotic cells, respectively. |
| Animal experiment [2,3]: | |
|
Animal models |
Medulloblastoma and pancreatic carcinoma xenografts mouse models, Male Sprague-Dawley rats with gastroesophageal reflux disease |
|
Dosage form |
Oral administration, 10 mg/kg |
|
Application |
In medulloblastoma and pancreatic carcinoma xenografts animal models, administration of BMS-833923 at single oral dose showed robust inhibition of Hh pathway. In a rat model with gastroesophageal reflux disease, administration of BMS-833923 (10 mg/kg/day) resulted in the decreased development of both Barrett esophagus and esophageal adenocarcinoma by 35.7%. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Zaidi A H, Komatsu Y, Kelly L A, et al. Smoothened inhibition leads to decreased proliferation and induces apoptosis in esophageal adenocarcinoma cells. Cancer investigation, 2013, 31(7): 480-489. [2]. Gendreau S B, Hawkins D, Ho C P, et al. Abstract B192: Preclinical characterization of BMS‐833923 (XL139), a hedgehog (HH) pathway inhibitor in early clinical development. Molecular Cancer Therapeutics, 2009, 8(12 Supplement): B192-B192. [3].Gibson M K, Zaidi A H, Davison J M, et al. Prevention of Barrett esophagus and esophageal adenocarcinoma by smoothened inhibitor in a rat model of gastroesophageal reflux disease. Annals of surgery, 2013, 258(1): 82-88. | |
| Description | BMS-833923 is an orally bioavailable small-molecule inhibitor of Smoothened with IC50 values of 6-35 nM. | |||||
| Targets | Smoothened | |||||
| IC50 | 6-35 nM | |||||
Quality Control & MSDS
- View current batch:
Chemical structure