ferritin heavy chain fragment [Multiple species]
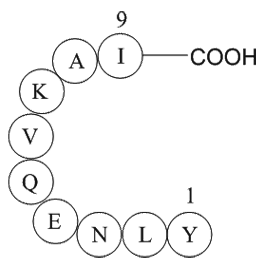
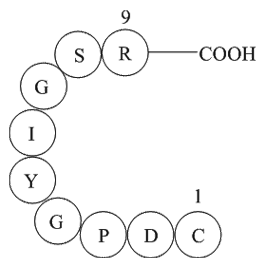
Sequence: H2N-YLNEQVKAI-OH
Ferritin is a protein of 450 kDa consisting of 24 subunits that is present in every cell type. In vertebrates, these subunits are both the light (L) and the heavy (H) type with an apparent molecular weight of 19 kDA or 21 kDA respectively; their sequences are about 50% homologous. [1] Heart, kidney, and placental ferritins are highly acidic because they are composed of mostly H chains [2] . The ferritin found in cancer cells was found to consist mainly of H chains [3][4].
The heavy chain of Ferritin also possesses ferroxidase activity, this involves the conversion of iron from the ferrous (Fe2+) to ferric (Fe 3+) forms. This limits the deleterious reaction which occurs between ferrous iron and hydrogen peroxide known as the Fenton reaction which produces the highly damaging hydroxyl radical.
If the ferritin level is low, there is a risk for lack of iron, which could lead to anemia.[5] If ferritin is high, there is iron in excess or else there is an acute inflammatory reaction in which ferritin is mobilized without iron excess. For example, ferritins may be high in infection without signalling body iron overload. Ferritin is also used as a marker for iron overload disorders, such as hemochromatosis or hemosiderosis.[6] Recent research has shown that H-Ferritin from melanoma cells may suppress immune responses.[7]
Ref:
1. Theil, E. (1987). "Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms." Annual review of biochemistry 56 (1): 289–315. doi:10.1146/annurev.bi.56.070187.001445.PMID 3304136.
2. Morikawa K., Oseko F., Morikawa S. A role for ferritin in hematopoiesis and the immune system. Leuk. Lymphoma, 18: 429-433, 1995.
3. Hazard J. T., Drysdale J. W. ferritinaemia in cancer. Nature (Lond.), 265:755-756, 1977.
4. Constanzo F., Colombo M., Staempfli S., Santoro C., Marone M., Frank R., Delius H., Cortese R. Structure of gene and pseudogenes of human apoferritin H.Nucleic Acids Res., 14: 721-736, 1986.
5. Guyatt G, Patterson C, Ali M, Singer J, Levine M, Turpie I, Meyer R (1990). "Diagnosis of iron-deficiency anemia in the elderly". Am J Med 88 (3): 205–9.doi:10.1016/0002-9343(90)90143-2. PMID 2178409.
6. Kennedy A, Kohn M, Lammi A, Clarke S (2004). "Iron status and haematological changes in adolescent female inpatients with anorexia nervosa". J Paediatr Child Health 40 (8): 430–2. doi:10.1111/j.1440-1754.2004.00432.x.PMID 15265182.
7. Gray C. P., Franco A. V., Arosio P., Hersey P. Immunosuppressive effects of melanoma-derived heavy-chain ferritinare dependent on stimulation of IL-10 production. Int. J. Cancer, 92: 843-850, 2001.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 1077.23 |
| Formula | C49H80N12O15 |
| Synonyms | H2N-Tyr-Leu-Asn-Glu-Gln-Val-Lys-Ala-Ile-OH |
| Solubility | ≥107.7 mg/mL in DMSO; insoluble in EtOH; ≥9.42 mg/mL in H2O with ultrasonic |
| SDF | Download SDF |
| Canonical SMILES | NC(CC1=CC=C(O)C=C1)C(NC(CC(C)C)C(NC(CC(N)=O)C(NC(CCC(O)=O)C(NC(CCC(N)=O)C(NC(C(C)C)C(NC(CCCCN)C(NC(C)C(NC(C(C)CC)C(O)=O)=O)=O)=O)=O)=O)=O)=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure
![ferritin heavy chain fragment [Multiple species]](/media/diy/images/struct/A1069.png)