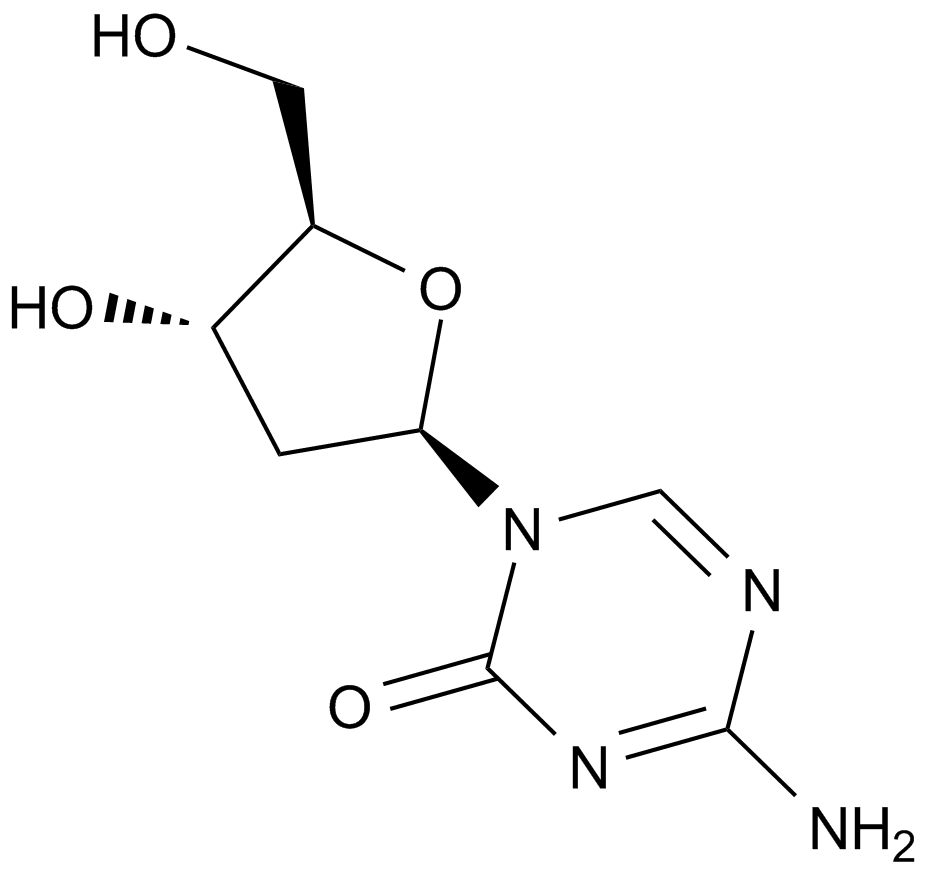
Decitabine (NSC127716, 5AZA-CdR)
Decitabine (CAS No. 2353-33-5) is a nucleoside analog DNA methyltransferase (DNMT) inhibitor, with DNMT1 (a key enzyme maintaining DNA methylation) as its primary therapeutic target. At the cellular level, its IC₅₀ ranges from 10 to 100 nM; low doses (10~100 nM) mainly exert immunomodulatory effects, while high doses (≥1 μM) exhibit cytotoxicity. Clinically, it is indicated for the treatment of intermediate-risk to high-risk myelodysplastic syndromes (MDS) based on the International Prognostic Scoring System (IPSS). The intravenous dosage is 15 mg/m² once daily for 5 consecutive days as one cycle (repeated every 4 weeks). Additionally, it can be used at low doses in combination with anti-PD-1 antibodies for the treatment of relapsed/refractory classical Hodgkin lymphoma and advanced solid tumors (e.g., gastric cancer, esophageal cancer), which can reverse immunotherapy resistance with favorable safety profiles and no significant myelosuppression.
Decitabine increases the expression of γ-globulin through a post-transcriptional mechanism independent of DNA methylation, thereby enabling its incorporation into DNA and formation of irreversible covalent bonds with DNA methyltransferases at cytosine sites targeted for DNA methylation. It has been reported that decitabine possesses substantial efficacy in reactivating epigenetically silenced tumor suppressor genes. In colorectal cancer cell lines HCT116 and RKO, decitabine increases the ratio of acetylation of histone H3 lysine 9 (H3K9ac) to methylation on the unmethylated promoters of hMLH1 and MGMT, respectively. In T24 bladder cancer cells, decitabine can enhance the acetylation of histone H3 lysine 9 and methylation of histone H3 lysine 4 (H3K4me) on the unmethylated p14 promoter.
References:
[1] Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, Bayar E, Lyons J, Rosenfeld CS, Cortes J, Kantarjian HM. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004 Mar 1;103(5):1635-40. doi: 10.1182/blood-2003-03-0687. Epub 2003 Nov 6. PMID: 14604977.
[2] Scott SA, Dong WF, Ichinohasama R, Hirsch C, Sheridan D, Sanche SE, Geyer CR, Decoteau JF. 5-Aza-2'-deoxycytidine (decitabine) can relieve p21WAF1 repression in human acute myeloid leukemia by a mechanism involving release of histone deacetylase 1 (HDAC1) without requiring p21WAF1 promoter demethylation. Leuk Res. 2006 Jan;30(1):69-76. doi: 10.1016/j.leukres.2005.05.010. Epub 2005 Jul 25. PMID: 16043219.
[3] Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, Faderl S, Bueso-Ramos C, Ravandi F, Estrov Z, Ferrajoli A, Wierda W, Shan J, Davis J, Giles F, Saba HI, Issa JP. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007 Jan 1;109(1):52-7. doi: 10.1182/blood-2006-05-021162. Epub 2006 Aug 1. PMID: 16882708.
[4] Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008 Jul 1;123(1):8-13. doi: 10.1002/ijc.23607. PMID: 18425818.
[5] Han P, Hou Y, Zhao Y, Liu Y, Yu T, Sun Y, Wang H, Xu P, Li G, Sun T, Hu X, Liu X, Li L, Peng J, Zhou H, Hou M. Low-dose decitabine modulates T-cell homeostasis and restores immune tolerance in immune thrombocytopenia. Blood. 2021 Aug 26;138(8):674-688. doi: 10.1182/blood.2020008477. PMID: 33876188; PMCID: PMC8394906.
[6] Li X, Li Y, Dong L, Chang Y, Zhang X, Wang C, Chen M, Bo X, Chen H, Han W, Nie J. Decitabine priming increases anti-PD-1 antitumor efficacy by promoting CD8+ progenitor exhausted T cell expansion in tumor models. J Clin Invest. 2023 Apr 3;133(7):e165673. doi: 10.1172/JCI165673. PMID: 36853831; PMCID: PMC10065084.
- 1. Dandan Li, Zeng Zhou, Xinqi Li. "Hypermethylation-mediated HNF4A silencing by Helicobacter pylori infection drives gastric cancer by disrupting epithelial cell polarity and activating EMT signaling." Cell Death Dis. 2025 Oct 6;16(1):688 PMID: 41053012
- 2. Dandan Li, Lingyun Xia, et al. "A new high-throughput screening methodology for the discovery of cancer-testis antigen using multi-omics data." Comput Methods Programs Biomed. 2024 Jun:250:108193 PMID: 38678957
- 3. Lei Lv, Qinqin Wei, et al. "IGF2BP3 prevent HMGB1 mRNA decay in bladder cancer and development." Cell Mol Biol Lett. 2024 Mar 19;29(1):39 PMID: 38504159
- 4. Dandan Li, Lingyun Xia, et al. "Serine protease PRSS56, a novel cancer-testis antigen activated by DNA hypomethylation, promotes colorectal and gastric cancer progression via PI3K/AKT axis." Cell Biosci. 2023 Jul 3;13(1):124 PMID: 37400936
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 228.08 |
| Cas No. | 2353-33-5 |
| Formula | C8H12N4O4 |
| Synonyms | 5-Aza-2'-deoxycytidine; Decitabine |
| Solubility | insoluble in EtOH; ≥11.4 mg/mL in DMSO; ≥23.3 mg/mL in H2O with gentle warming |
| Chemical Name | 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2-one |
| SDF | Download SDF |
| Canonical SMILES | NC(N=CN1[C@@H](C2)O[C@H](CO)[C@H]2O)=NC1=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Human and murine melanoma cells (A375 and B16). |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
Decitabine 0.5 μM added on Days 1 and 4 and images are obtained on Day 8. |
|
Applications |
Decitabine decreases melanoma cell line proliferation and induces morphologic changes of differentiation. |
| Animal experiment [2]: | |
|
Animal models |
Mice bearing U2OS xenografts. |
|
Dosage form |
2.5 mg/kg intraperitoneally on Days 29, 31 and 33. On Day 37, mice are sacrificed. |
|
Preparation method |
Dissolved in saline (0.9% w/v NaCl). |
|
Applications |
Decitabine significantly reduces tumor xenograft size and lowers mitotic activity, increases the amount of apoptotic cells and bone matrix production. Decitabine also increases the expression of GADD45A, HSPA9B, PAWR, PDCD5, NFKBIA, and TNFAIP3 to ≥2-fold, which are pro-apoptotic genes [2]. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Alcazar O, Achberger S, Aldrich W, et al. Epigenetic regulation by decitabine of melanoma differentiation in vitro and in vivo. Int J Cancer, 2012, 131 (1): 18-29. [2] Al-Romaih K, Somers GR, Bayani J, et al. Modulation by decitabine of gene expression and growth of osteosarcoma U2OS cells in vitro and in xenografts: identification of apoptotic genes as targets for demethylation. Cancer Cell Int, 2007, 7: 14. |
|
Quality Control & MSDS
- View current batch:
Chemical structure