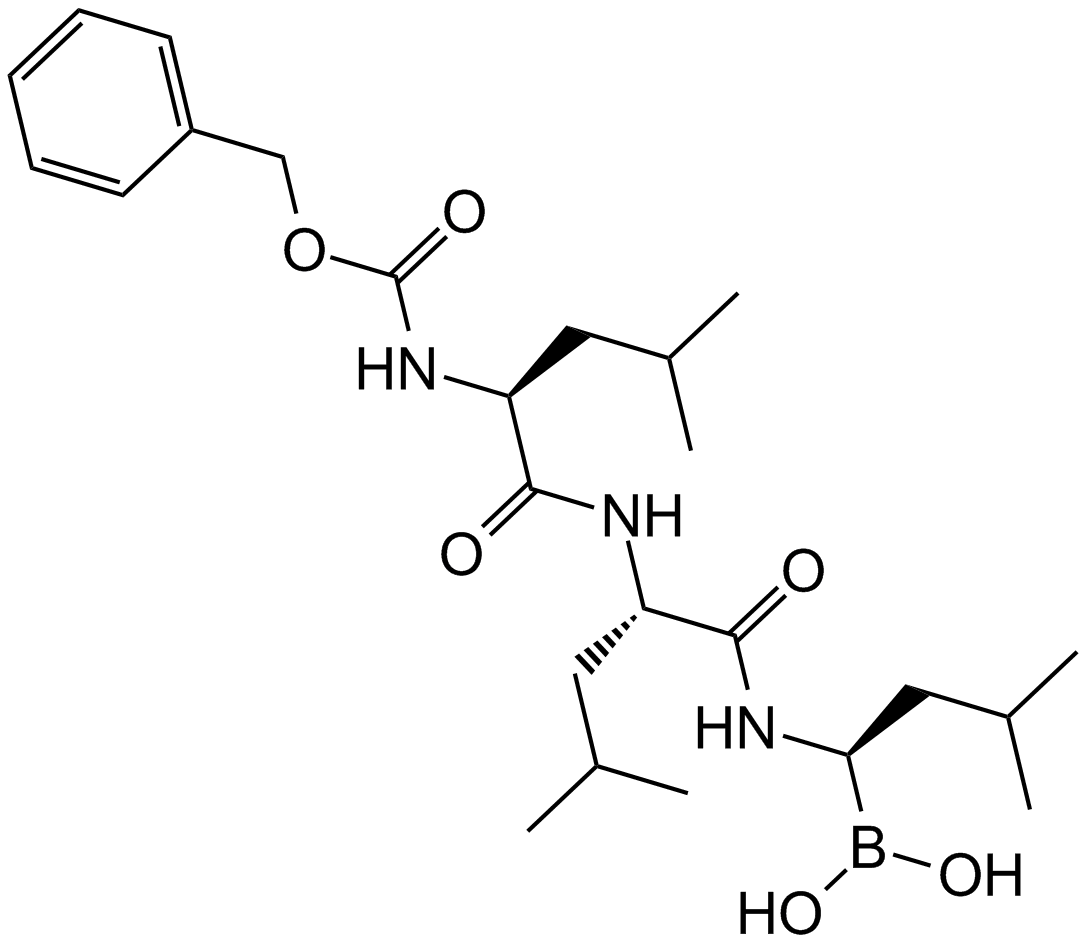
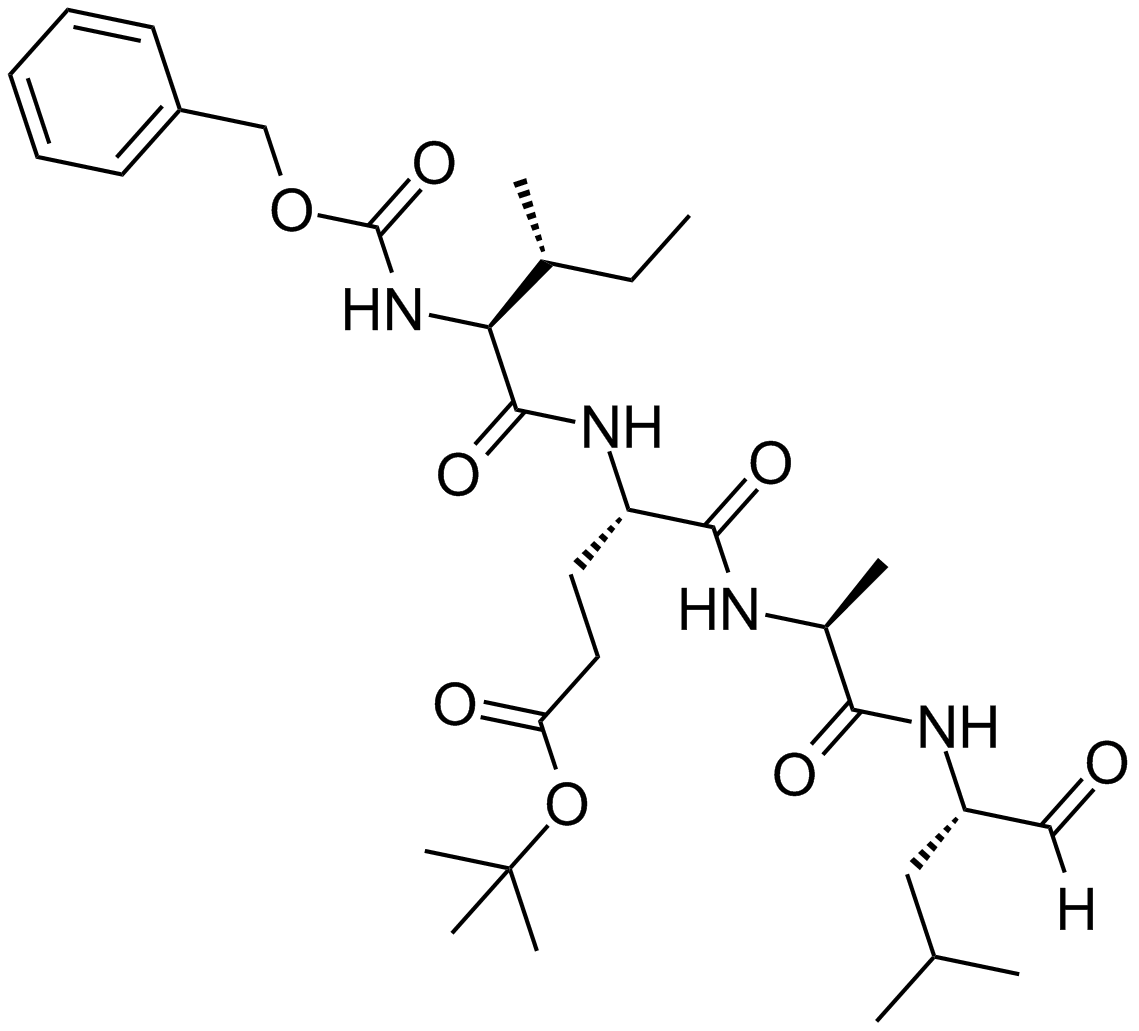
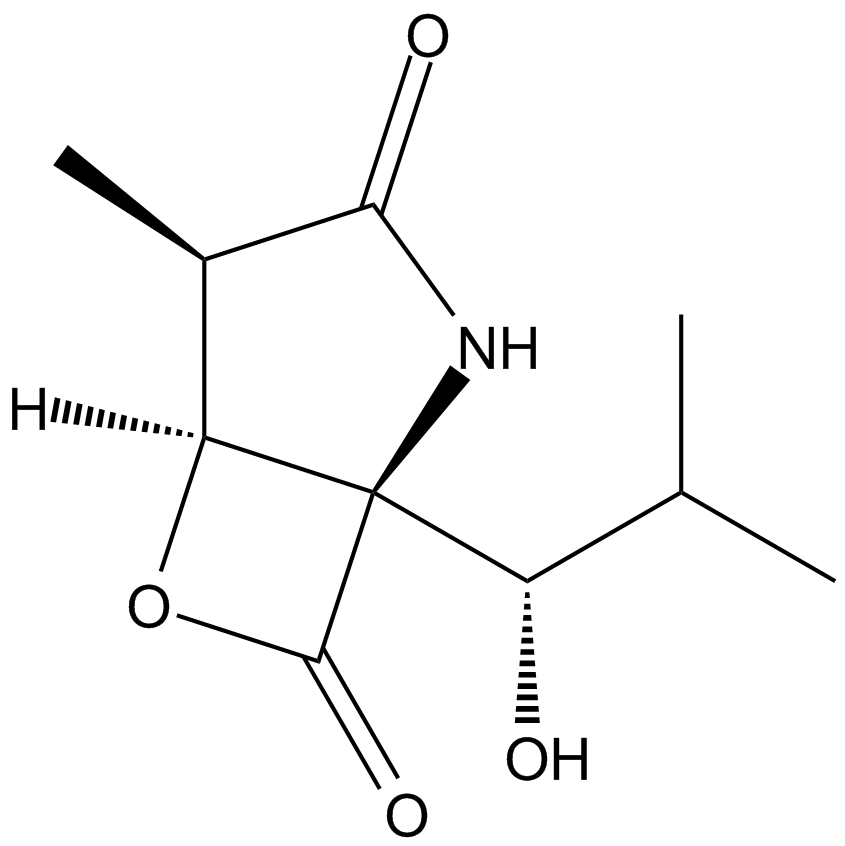
Salinosporamide A (NPI-0052, Marizomib)
Salinosporamide A is a potent inhibitor of 20S proteasome with IC50 value of 1.3 nM [1].
Salinosporamide A was isolated from the crude extract of a Salinospora strain CNB-392. It showed potent anti-tumor activity with an IC50 value of 11 ng/mL in HCT-116 cells. It also exerted a mean GI50 value of less than 10 nM in the NCI’s 60 cell line-panel. Among these cell lines, Salinosporamide A showed the greatest potent efficacies in NCI-H226, SF-539, SK-MEL-28 and MDA-MB-435 cells. Salinosporamide A inhibited the purified 20S proteasome with IC50 value of 1.3 nM. It was about 35-fold more potent than the first discovered specific proteasome inhibitor, omuralide [1].
References:
[1] Feling R H, Buchanan G O, Mincer T J, et al. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angewandte Chemie International Edition, 2003, 42(3): 355-357.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 313.78 |
| Cas No. | 437742-34-2 |
| Formula | C15H20ClNO4 |
| Synonyms | salinosporamide A, MARIZOMIB, NPI-0052, (-)-Salinosporamide A |
| Solubility | Soluble in DMSO |
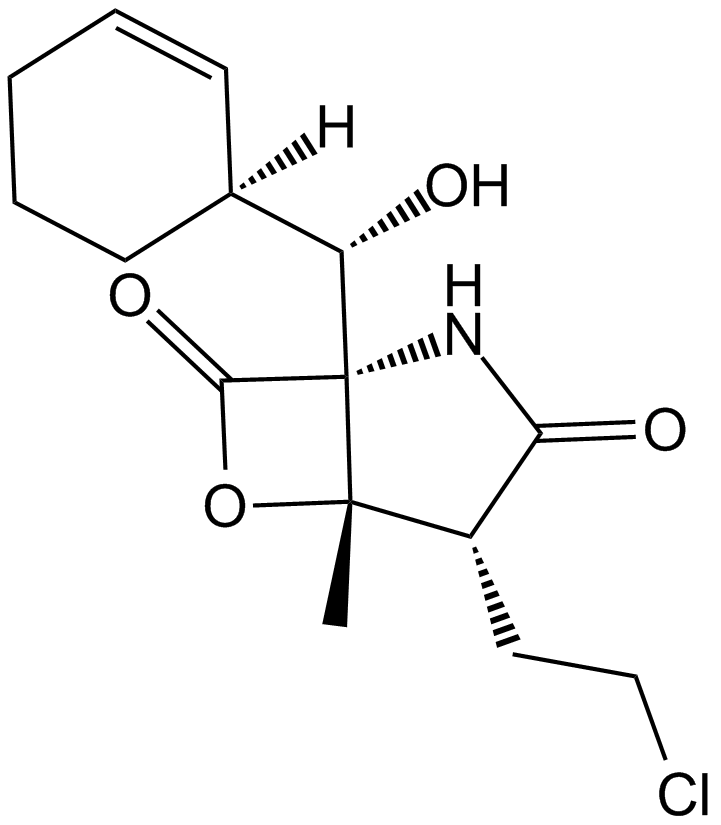
| Chemical Name | (1S,2R,5R)-2-(2-chloroethyl)-5-[(S)-[(1S)-cyclohex-2-en-1-yl]-hydroxymethyl]-1-methyl-7-oxa-4-azabicyclo[3.2.0]heptane-3,6-dione |
| SDF | Download SDF |
| Canonical SMILES | C[C@]([C@H]1CCCl)([C@@]2([C@H]([C@@H]3C=CCCC3)O)NC1=O)OC2=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Human MM-cell lines (MM.1S, INA-6, RPMI-8226, MM.1R,KMS12PE, and U266) |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
24 h; 2nM |
|
Applications |
Human MM-cell lines were pretreated with lenalidomide for 24 hours; NPI-0052 was then added for an additional 24 hours, followed by assessment for cell viability using MTT assays. A significant decrease in viability of all cell lines was observed in response to treatment with combined low doses of NPI-0052 and lenalidomide compared with either agent alone(P<0.05; n=3). These data demonstrate synergistic anti-MM activity of NPI-0052 plus lenalidomide. |
| Animal experiment [2]: | |
|
Animal models |
CB-17 SCID-male mice |
|
Dosage form |
0.15 mg/kg; i.v. |
|
Applications |
MM.1S-tumour bearing mice were injected with NPI-0052(0.15 mg/kg; i.v.) twice a week for 3 weeks, and tumour volume was measured. NPI-0052 treatment significantly decreased tumour growth relative to vehicle-treated control mice (P =0.005). NPI-0052 treatment was not associated with any toxicity, because no differences in body weight and overall appearance were noted. Importantly, the anti-MM activity of NPI-0052 was evident as early as day 5–7, when significant proteasome inhibition was observed in the tumours. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Chauhan D, Singh A V, Ciccarelli B, et al. Combination of novel proteasome inhibitor NPI-0052 and lenalidomide trigger in vitro and in vivo synergistic cytotoxicity in multiple myeloma[J]. Blood, 2010, 115(4): 834-845. [2] Singh A V, Palladino M A, Lloyd G K, et al. Pharmacodynamic and efficacy studies of the novel proteasome inhibitor NPI‐0052 (marizomib) in a human plasmacytoma xenograft murine model[J]. British journal of haematology, 2010, 149(4): 550-559. | |
| Description | Salinosporamide A (NPI-0052, Marizomib) is a novel inhibitor of marine derived proteasome which inhibits CT-L, C-L, and T-L proteasome activities in human erythrocyte-derived 20S proteasomes with EC50 values of 3.5 nM, 430 nM, 28 nM, respectively. | |||||
| Targets | CT-L (EC50) | C-L (EC50) | T-L (EC50) | |||
| IC50 | 3.5 nM | 430 nM | 28 nM | |||
Quality Control & MSDS
- View current batch:
Chemical structure