NS 11394
NS 11394 is a selective GABA(A) receptor-positive modulator.
Preclinical studies suggest that GABAA-1, 2, and 5-containing receptors can mediate the sedative/ motor-impairing, anxiolytic, and memory impairing effects, respectively.
In vitro: Previous study showed that NS11394 possessed a functional selectivity profile at GABA(A) receptors of alpha(5) > alpha(3) > alpha(2) > alpha(1) based on oocyte electrophysiology with human GABA(A) receptors. Moreover, compared with other subtype-selective ligands, NS11394 was unique in having superior efficacy for GABA(A)-alpha(3) receptors while maintaining low efficacy for GABA(A)-alpha(1) receptors [1].
In vivo: Animal study showed that NS11394 had an excellent PK profile, which correlated with pharmacodynamic endpoints of CNS receptor occupancy. In addition, it was showd that NS11394 was potent and highly effective in rodent anxiety models. The anxiolytic efficacy of NS11394 was most probably mediated via its high efficacy at GABA(A)-alpha(3) receptors, though the contribution of GABA(A)-alpha(2) receptors could not be excluded. Moreover, when compared with benzodiazepines, NS11394 had a significantly reduced side effect profile in rat and mouse, even at full CNS receptor occupancy. The authors attributed such benign side effect profile to very low efficacy of NS11394 at GABA(A)-alpha(1) receptors and a partial agonist profile of receptor subtypes. It was also found that NS11394 could impair memory in both rats and mice, which was possibly attributable to its efficacy at GABA(A)-alpha(5) receptors [1].
Clinical trial: Up to now, NS 11394 is still in the preclinical development stage.
Reference:
[1] Mirza NR, et al. NS11394 [3'-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl- 2-carbonitrile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther. 2008 Dec;327(3):954-68.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 353.42 |
| Cas No. | 951650-22-9 |
| Formula | C23H19N3O |
| Solubility | ≥35.3 mg/mL in DMSO with gentle warming; insoluble in H2O; ≥11.02 mg/mL in EtOH |
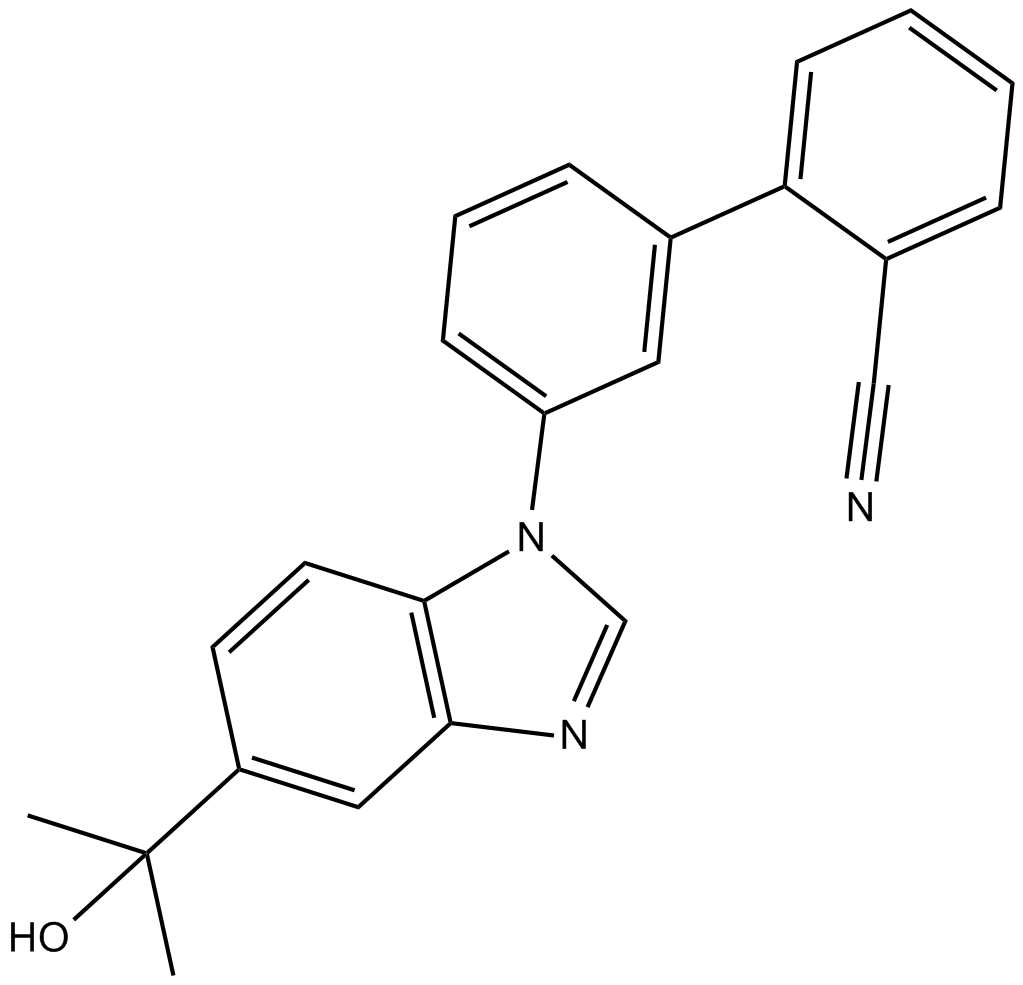
| Chemical Name | 3'-(5-(2-hydroxypropan-2-yl)-1H-benzo[d]imidazol-1-yl)-[1,1'-biphenyl]-2-carbonitrile |
| SDF | Download SDF |
| Canonical SMILES | CC(C)(c(cc1)cc(nc2)c1[n]2-c1cc(-c(cccc2)c2C#N)ccc1)O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure