MDV3100 (Enzalutamide)
MDV3100, known as Enzalutamide, is a second-generation androgen receptor (AR) signaling inhibitor. It has been demonstrated impressive affinity to the AR compared to the first-generation AR inhibitors. It is able to inhibit binding of androgens to the AR, AR nuclear translocation, and the association of the AR with DNA. The AR is a 919-amino acid member of the steroid receptor transcription factor superfamily with different domains including an N-terminal regulation domain, a central DNA binding domain, and a C-terminal domain, which includes the ligand-binding domain incorporated within its protein structure. MDV3100 was identified by the Sawyers/Jung laboratories by using the nonsteroidal agonist. Testing was showing that it induced apoptosis in VCaP cells, an AR gene amplified human prostate cancer line, while bicalutamide was ineffective.
Reference
Howard I. Scher, Karim Fizazi, Fred Saad, Mary-Ellen Taplin, Cora N. Sternberg, Kurt Miller, Ronald De Wit, Peter Mulders, Mohammad Hirmand, Bryan Selby, Johann Sebastian. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. Journal of Clinical Oncology. 2012; 30(5):
Manoj P. Menon, Celestia S. Higano. Enzalutamide, a Second Generation Androgen Receptor Antagonist: Development and Clinical Applications in Prostate Cancer. Current Oncology Reports. 2013; 15(2): 69 – 75.
Joelle El-Amm, Nihar Patel, Ashley Freeman, Jeanny B. Aragon-Ching. Metastatic Castration-Resistant Prostate Cancer: Critical Review of Enzalutamide. Clinical Medicine Insights: Oncology. 2013; 7: 235 – 245.
- 1. Bianca Calì, Martina Troiani, et al. "Coagulation factor X promotes resistance to androgen-deprivation therapy in prostate cancer." Cancer Cell. 2024 Sep 16:S1535-6108(24)00317-9 PMID: 39303726
- 2. Xiaofeng Cheng, Heng Yang, et al. "METTL3-mediated m6A modification of circGLIS3 promotes prostate cancer progression and represents a potential target for ARSI therapy." Cell Mol Biol Lett. 2024 Aug 14;29(1):109 PMID: 39143552
- 3. Wenliang Li, Dayong Zheng, et al. "Androgen deprivation induces neuroendocrine phenotypes in prostate cancer cells through CREB1/EZH2-mediated downregulation of REST." Res Sq. 2023 Oct 4:rs.3.rs-3270539. PMID: 37886478
- 4. K M Biernacka, R Barker, et al. "A role for androgen receptor variant 7 in sensitivity to therapy: Involvement of IGFBP-2 and FOXA1." Transl Oncol. 2023 Aug:34:101698. PMID: 37307644
- 5. Justin M. Silverman. "CHARACTERIZING THE EFFECTS OF ANTIANDROGENS AND SENOLYTICS TO ENHANCE THE THERAPEUTIC RESPONSE TO CASTRATION-RESISTANT PROSTATE CANCER." VCU Scholars Compass. August 02, 2023.
- 6. Nicolò Bancaro, Bianca Calì, et al. "Apolipoprotein E induces pathogenic senescent-like myeloid cells in prostate cancer." Cancer Cell. 2023 Mar 13;41(3):602-619.e11. PMID: 36868226
- 7. Belal M Ali, Hanan S El-Abhar, et al. "Blocking Androgen Receptor/Androgen Receptor Variant7 Decreases Metastasis of Estrogen Receptor Positive Breast Cancer Cells Through Modulating Epithelial to Mesenchymal Transition." Research Square. March 15th, 2022.
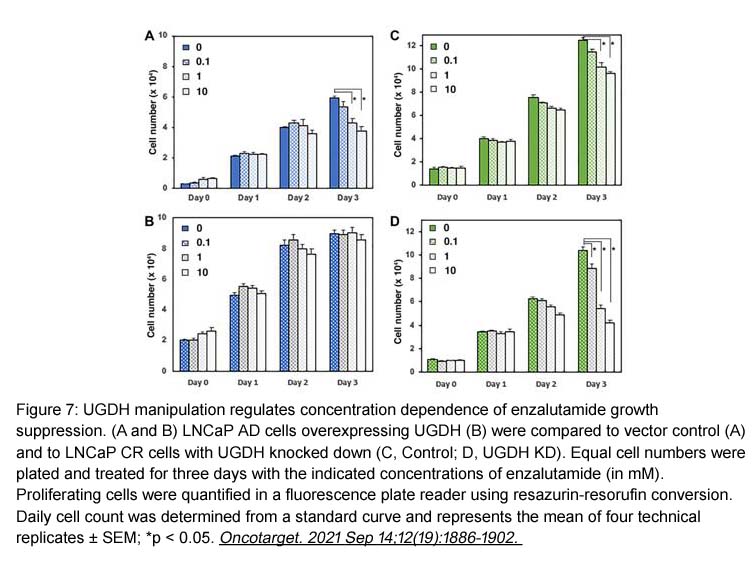
- 8. Brenna M. Zimmer, Michelle E. Howell, et al. "Altered glucuronidation deregulates androgen dependent response profiles and signifies castration resistance in prostate cancer." Oncotarget. 2021 Sep 14;12(19):1886-1902. PMID: 34548906
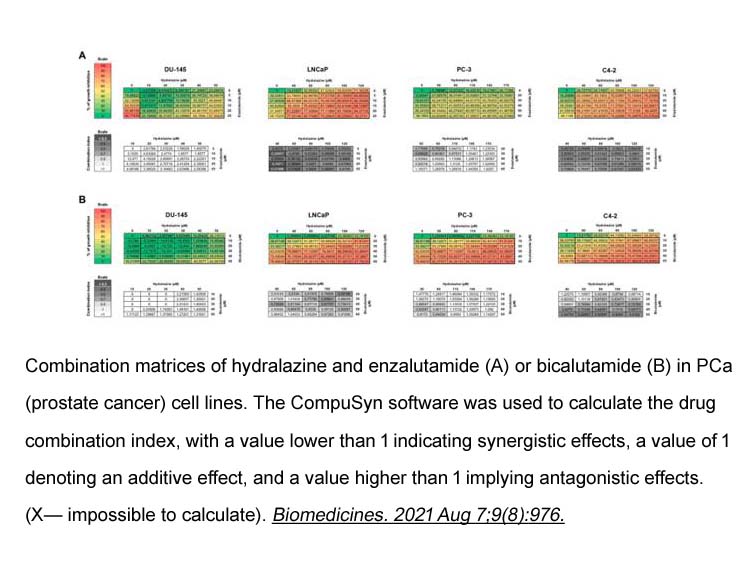
- 9. Nair Lopes, Mariana Brütt Pacheco, et al. "Hydralazine and Enzalutamide: Synergistic Partners against Prostate Cancer." Biomedicines. 2021 Aug 7;9(8):976. PMID: 34440180
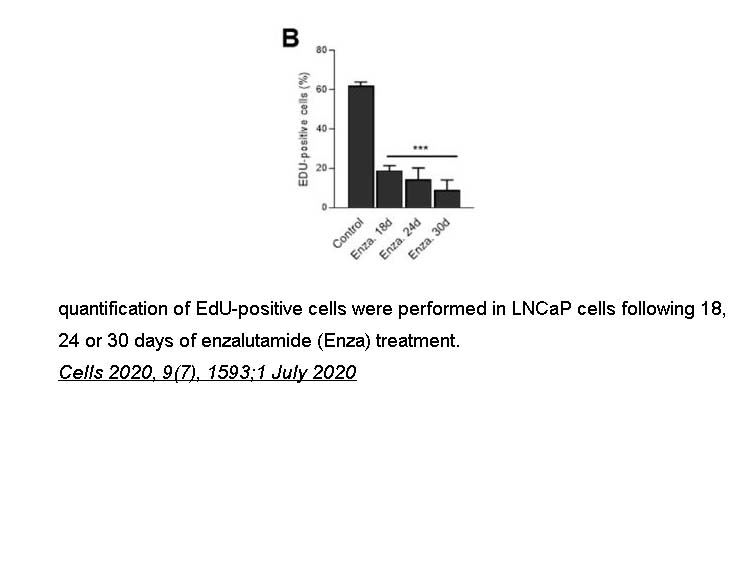
- 10. Nicolas Malaquin, Arthur Vancayseele, et al. "DNA Damage- But Not Enzalutamide-Induced Senescence in Prostate Cancer Promotes Senolytic Bcl-xL Inhibitor Sensitivity." Cells 2020, 9(7), 1593;1 July 2020. PMID: 32630281
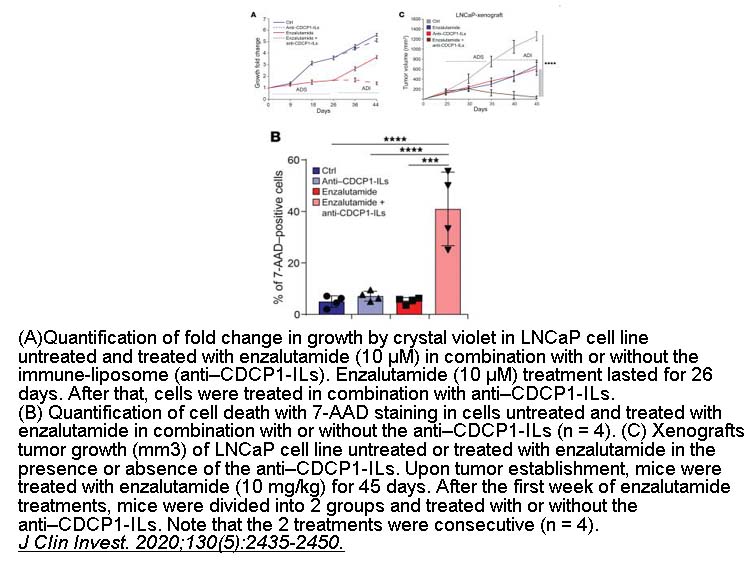
- 11. Alajati A, D'Ambrosio M, et al. "CDCP1 overexpression drives prostate cancer progression and can be targeted in vivo." J Clin Invest. 2020;130(5):2435-2450. PMID: 32250342
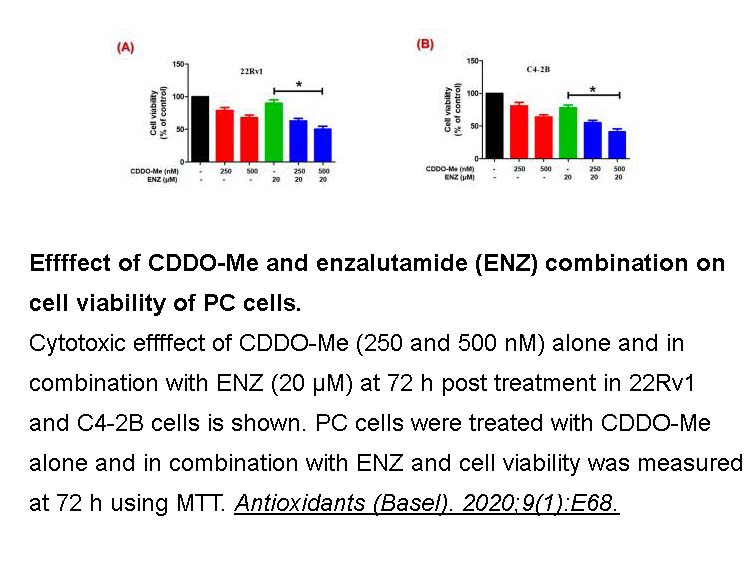
- 12. Khurana N, Chandra PK, et al. "Bardoxolone-Methyl (CDDO-Me) Suppresses Androgen Receptor and Its Splice-Variant AR-V7 and Enhances Efficacy of Enzalutamide in Prostate Cancer Cells." Antioxidants (Basel). 2020;9(1):E68. PMID: 31940946
- 13. PAIGE M. GLUMAC. "Targeting CD133 In Androgen Receptor Indifferent, Neuroendocrine Differentiated Aggressive Variant Prostate Cancer." UNIVERSITY OF MINNESOTA. 2019.
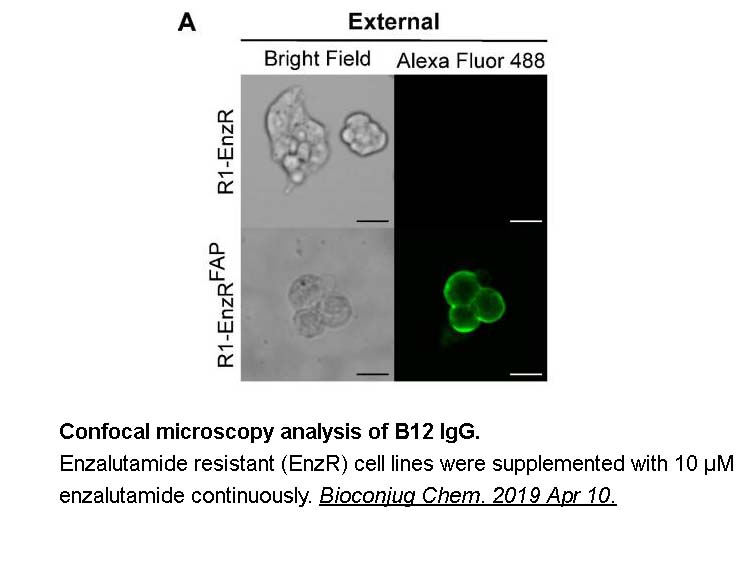
- 14. Hintz HM, Cowan AE, et al. "Development of a Cross-Reactive Monoclonal Antibody for Detecting the Tumor Stroma." Bioconjug Chem. 2019 May 15;30(5):1466-1476. PMID: 30966746
- 15. Zhang Y, Zheng D, et al. "Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers." Nat Commun. 2018 Oct 4;9(1):4080. PMID: 30287808
- 16. Li Q, Deng Q, et al. "Linking prostate cancer cell AR heterogeneity to distinct castration and enzalutamide responses." Nat Commun. 2018 Sep 6;9(1):3600. PMID: 30190514
- 17. Calcinotto A, Spataro C, et al. "IL-23 secreted by myeloid cells drives castration-resistant prostate cancer." Nature.2018 Jul;559(7714):363-369. PMID: 29950727
- 18. Khurana N, Kim H, et al. "Multimodal actions of the phytochemical sulforaphane suppress both AR and AR-V7 in 22Rv1 cells: Advocating a potent pharmaceutical combination against castration-resistant prostate cancer." Oncol Rep. 2017 Aug 30. PMID: 28901514
- 19. Audet-Walsh É, Dufour CR, et al. "Nuclear mTOR acts as a transcriptional integrator of the androgen signaling pathway in prostate cancer." Genes Dev. 2017 Jul 19. PMID: 28724614
- 20. Bao D, Cheng C, et al. "Regulation of p53wt glioma cell proliferation by androgen receptor-mediated inhibition of small VCP/p97-interacting protein expression."Oncotarget. 2017 Apr 4;8(14):23142-23154. PMID: 28423563
- 21. Sun J, Wang D, et al. "Androgen Receptor Regulates the Growth of Neuroblastoma Cells in vitro and in vivo." Front Neurosci. 2017 Mar 7;11:116. PMID: 28326012
- 22. Audet-Walsh É, Yee T, et al. "Androgen-Dependent Repression of ERRγ Reprograms Metabolism in Prostate Cancer." Cancer Res. 2017 Jan 15;77(2):378-389. PMID: 27821488
- 23. Khurana N, Talwar S, et al. "Sulforaphane increases the efficacy of anti-androgens by rapidly decreasing androgen receptor levels in prostate cancer cells." Int J Oncol. 2016 Oct;49(4):1609-19. PMID: 27499349
- 24. Wang L, Wang J, et al. "Co-targeting hexokinase 2-mediated Warburg effect and ULK1-dependent autophagy suppresses tumor growth of PTEN- and TP53-deficiency-driven castration-resistant prostate cancer." EBioMedicine. 2016 May;7:50-61. PMID: 27322458
- 25. Bogner J, Zolghadr K, et al. "The fluorescent two-hybrid assay for live-cell profiling of androgen receptor modulators." J Steroid Biochem Mol Biol. 2016 May 9. PMID: 27174722
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 464.4 |
| Cas No. | 915087-33-1 |
| Formula | C21H16F4N4O2S |
| Synonyms | Enzalutamide, MDV3100, MDV-3100, MDV 3100 |
| Solubility | ≥23.22 mg/mL in DMSO; insoluble in H2O; ≥9.44 mg/mL in EtOH |
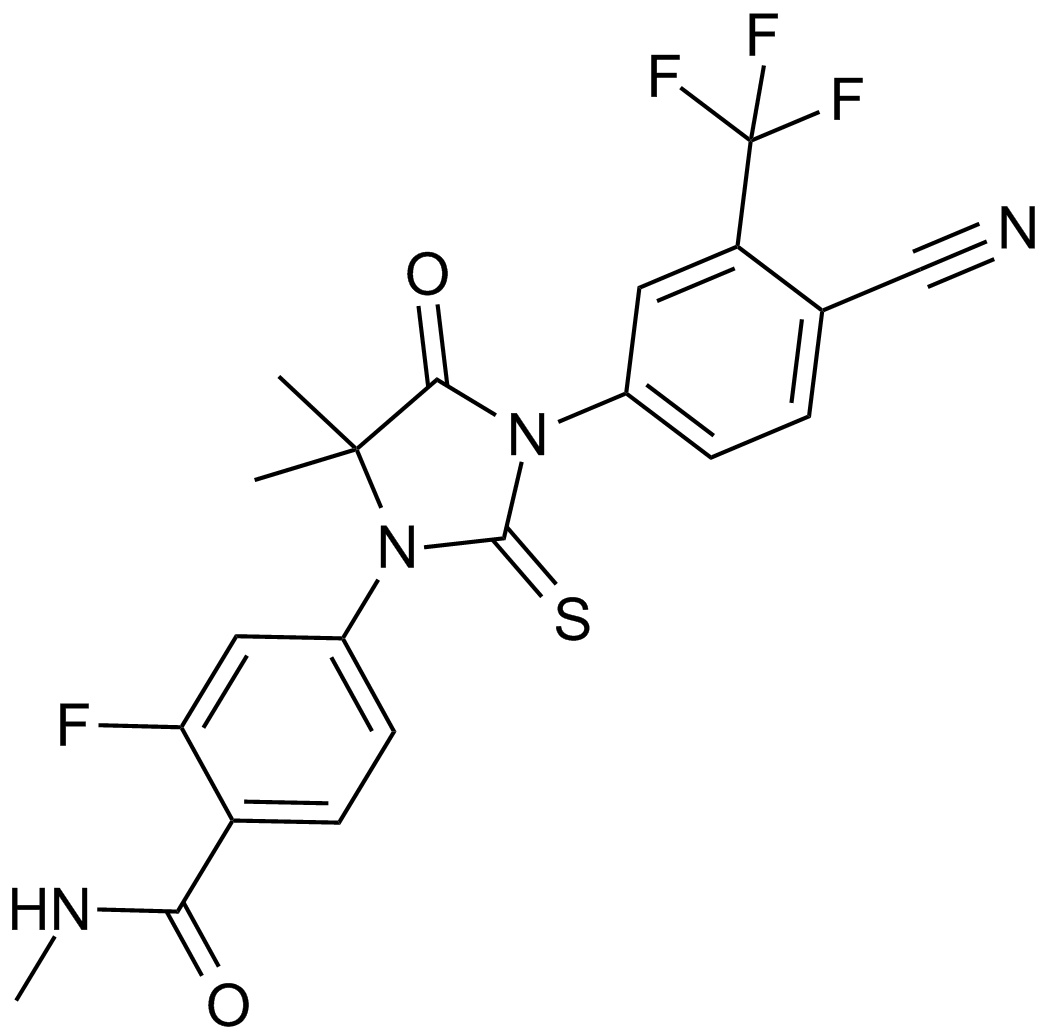
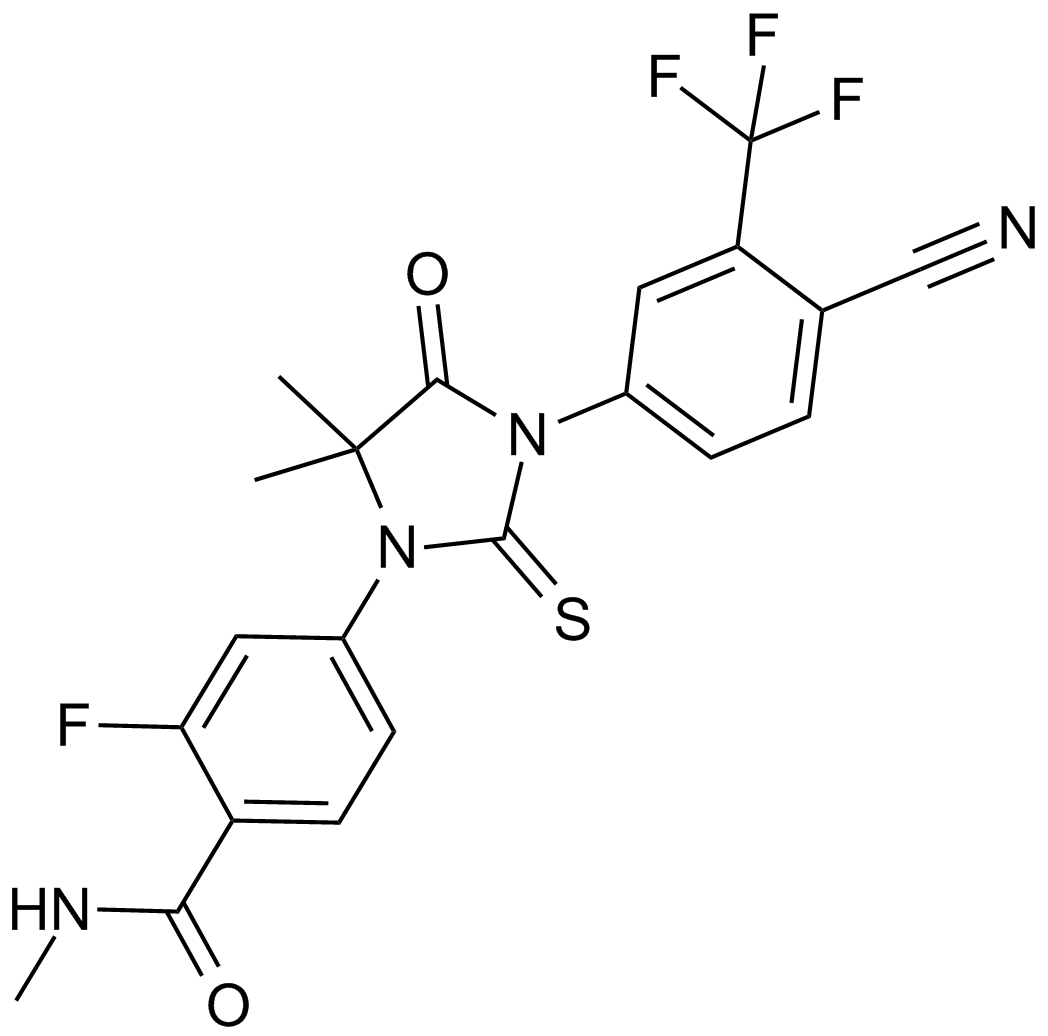
| Chemical Name | 4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl]-2-fluoro-N-methylbenzamide |
| SDF | Download SDF |
| Canonical SMILES | CC1(C(=O)N(C(=S)N1C2=CC(=C(C=C2)C(=O)NC)F)C3=CC(=C(C=C3)C#N)C(F)(F)F)C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[1] | |
|
Cell lines |
VCaP, LNCaP, 22RV1, DU145 and PC3 prostate cancer cell lines |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
10 μM,12h |
|
Applications |
Recruitment of AR to target loci was markedly attenuated by MDV3100 and less so by bicalutamide. Interestingly, JQ1 blocked AR recruitment almost as effectively as MDV3100. Limiting our evaluationto AR and BRD4 coincident peaks, we observed that DHT-mediated AR recruitment to these loci was inhibited by MDV3100 and to a lesser extent by JQ1. Corroborating the ChIP seq data, gene expression analysis in VCaP and LNCaP cells showed more efficient repression of DHT-induced AR-target genes by JQ1 than by MDV3100 or bicalutamide. |
| Animal experiment:[1] | |
|
Animal models |
Four-week-old male SCIDC.B17 mice |
|
Dosage form |
10 mg/kg,oral gavage or intraperitonially,five days a week |
|
Applications |
Treatment of VCaP tumour-bearing mice with JQ1 led to a significant reduction in tumour volume/weight, whereas MDV3100 had a less pronounced effect. Recently, several studies described the pro-metastatic effects of MDV3100 in pre-clinical models. To test whether MDV3100 treatment leads to spontaneous metastasis in our VCaP xenograft model, we isolated femur, liver and spleen from MDV3100-treated mice and found evidence of metastases in femur and liver. By contrast, JQ1-treated mice showed no evidence of metastasis. Taken together, these pre-clinical studies suggest that the use of MDV3100 in clinically localized prostate cancer may potentiate the formation of micro-metastases, unlike BET inhibitors. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| Phase III experiment [2]: | |
|
Target |
Men with castration-resistant prostate cancer |
|
Dosage form |
160 mg per day (800 patients) or placebo (399 patients) |
|
Applications |
The superiority of enzalutamide over placebo was shown with respect to all secondary end points: the proportion of patients with a reduction in the prostate-specific antigen (PSA) level by 50% or more (54% vs. 2%, P<0.001), the soft-tissue response rate (29% vs. 4%, P<0.001), the quality-of-life response rate (43% vs. 18%, P<0.001), the time to PSA progression (8.3 vs. 3.0 months; hazard ratio, 0.25; P<0.001), radiographic progression-free survival (8.3 vs. 2.9 months; hazard ratio, 0.40; P<0.001), and the time to the first skeletal-related event (16.7 vs. 13.3 months; hazard ratio, 0.69; P<0.001). Rates of fatigue, diarrhea, and hot flashes were higher in the enzalutamide group. |
|
References: 1. Asangani IA, Dommeti VL, Wang X et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014 Jun 12;510(7504):278-82. 2. Scher HI, Fizazi K, Saad F et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012 Sep 27;367(13):1187-97. |
|
| Description | Enzalutamide (MDV3100) is an antagonist of androgen-receptor (AR) with IC50 of 36 nM. | |||||
| Targets | Androgen-receptor | |||||
| IC50 | 36 nM | |||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
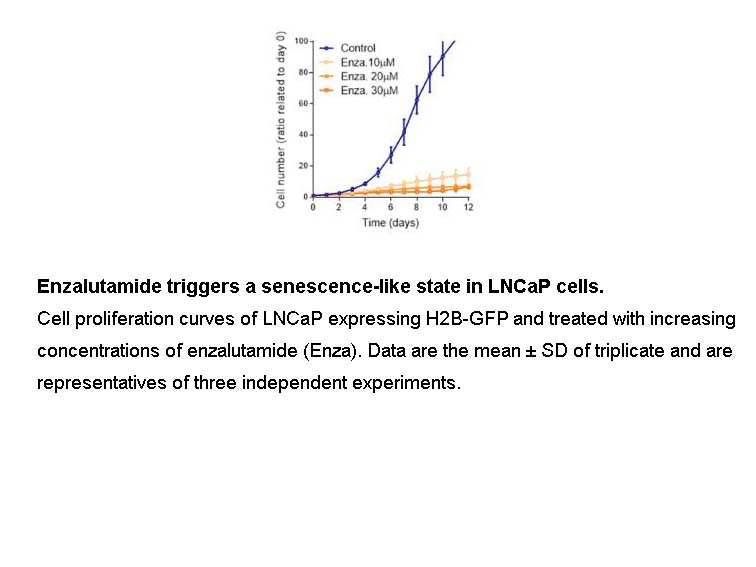
Related Biological Data