Influenza Hemagglutinin (HA) Peptide
HA tag,Complexes containing plasmid DNA, transferrin-polylysine conjugates, and polylysine-conjugated peptides derived from the N-terminal sequence of the influenza virus hemagglutinin subunit HA-2 have been used for the transfer of luciferase or -galactosidase marker genes to K562 cells, HeLa cells, and BNL CL.2 hepatocytes. The presence of these influenza peptide conjugates in the DNA complexes renders the complexes active in membrane disruption in a liposome leakage assay and results in a substantial augmentation of the transferrin-polylysine-mediated gene transfer.
Fusogenic peptides derived from the N-terminal sequence of the influenza virus hemagglutinin subunit HA-2 is part of the DNA complexes and the resulting augmentation of gene transfer to cultured cells1.
In the influenza virus, the hemagglutinin (HA) protein mediates both binding of the virus to the cell surface and the subsequent fusion of viral and cellular membranes. HA is composed of a receptor-binding subunit, denoted HA1, and a fusogenic subunit, denoted HA2. The native HA1yHA2 complex, as found on the surface of the native virus, is fusioninactive. For influenza virus, membrane fusion is regulated by the conformational state of the hemagglutinin (HA) protein, which switches from a native (nonfusogenic) structure to a fusion-active (fusogenic) conformation when exposed to the acidic environment of the cellular endosome. The native structure of HA is trapped in a metastable state and that the fusogenic conformation is released by destabilization of native structure2.
References:
1. E. WAGNER, C. PLANK et al, Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: Toward a synthetic virus-like gene-transfer vehicle. Proc. Nati. Acad. Sci. USA Vol. 89, pp. 7934-7938, September 1992
2. C. M. CARR, C. CHAUDHRY, P. S. KIM et al. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14306–14313
- 1. Zhewen Dong, Xiaofei She, et al. "The E3 ligase NEDD4L prevents colorectal cancer liver metastasis via degradation of PRMT5 to inhibit the AKT/mTOR signaling pathway." bioRxiv. October 24, 2024.
- 2. Jiang-Sha Zhao, Shuo Shi, et al. "Glutamine synthetase licenses APC/C-mediated mitotic progression to drive cell growth." Nat Metab. 2022 Feb;4(2):239-253. PMID: 35145325
- 3. Denghui Wei, Weixiang Zhan, et al. "RAB31 marks and controls an ESCRT-independent exosome pathway." Cell Res. 2021 Feb;31(2):157-177. PMID: 32958903
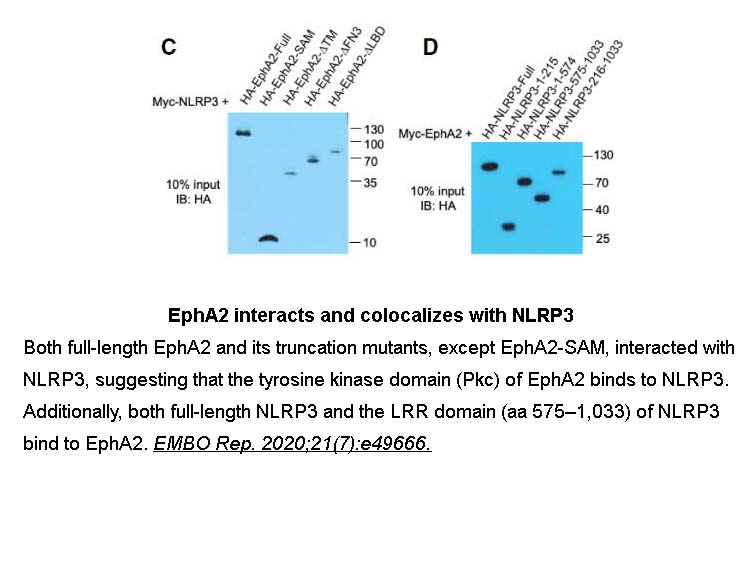
- 4. Zhang A, Xing J, et al. "EphA2 phosphorylates NLRP3 and inhibits inflammasomes in airway epithelial cells." EMBO Rep. 2020;21(7):e49666. PMID: 32352641
- 5. Ji R, Xu X, et al. "Regulation of adiponectin on lipid metabolism in large yellow croaker (Larimichthys crocea)." Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(8):158711. PMID: 32289502
- 6. Janani Subramaniam, Pu Yang, et al. "Identification and characterization of self-association domains on small ankyrin 1 isoforms." J Mol Cell Cardiol. 2020 Feb:139:225-237. PMID: 32035138
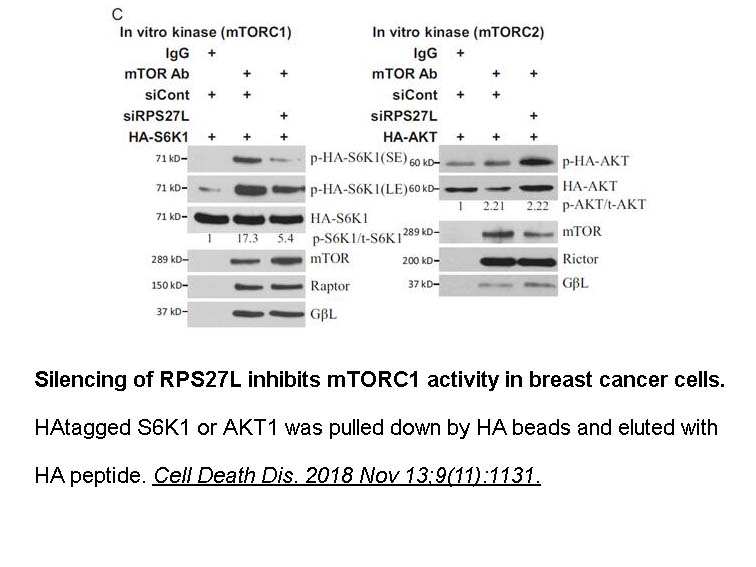
- 7. "Ribosomal protein S27-like regulates autophagy via the β-TrCP-DEPTOR-mTORC1 axis." Cell Death Dis. 2018 Nov 13;9(11):1131. PMID: 30425236
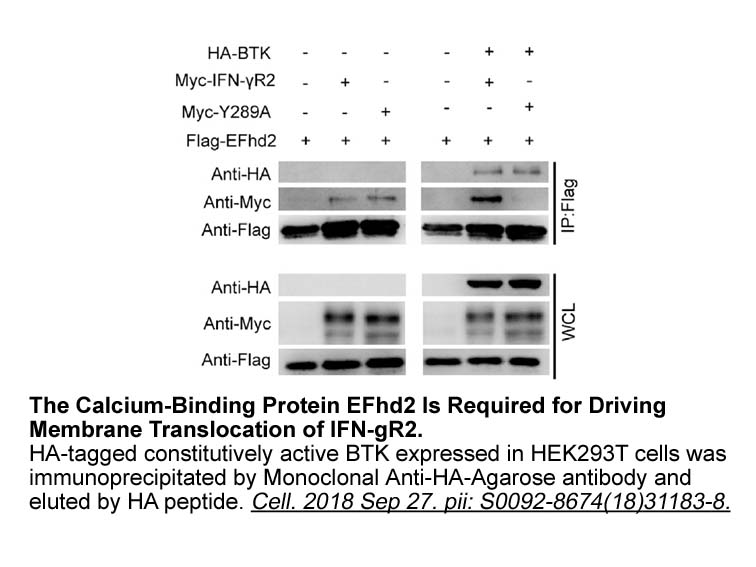
- 8. Xu X, Xu J, et al. "Phosphorylation-Mediated IFN-γR2 Membrane Translocation Is Required to Activate Macrophage Innate Response." Cell. 2018 Sep 27. pii: S0092-8674(18)31183-8. PMID: 30318148
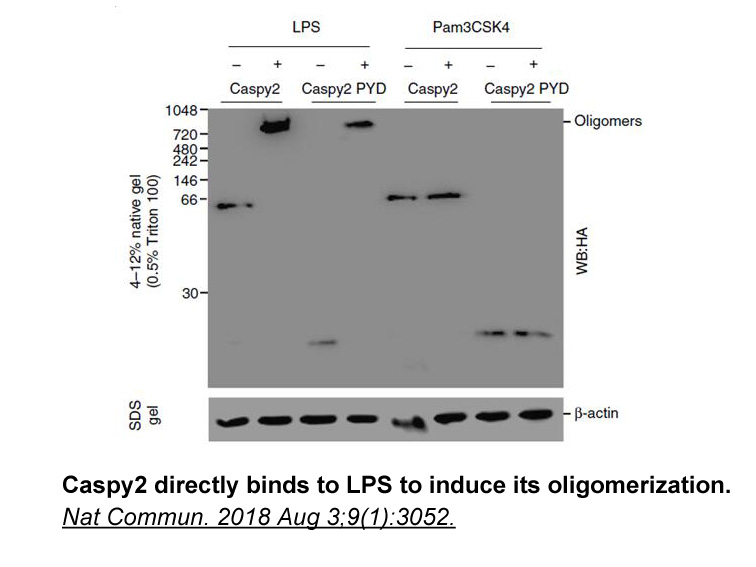
- 9. Yang D, Zheng X, et al. "Sensing of cytosolic LPS through caspy2 pyrin domain mediates noncanonical inflammasome activation in zebrafish." Nat Commun. 2018 Aug 3;9(1):3052. PMID: 30076291
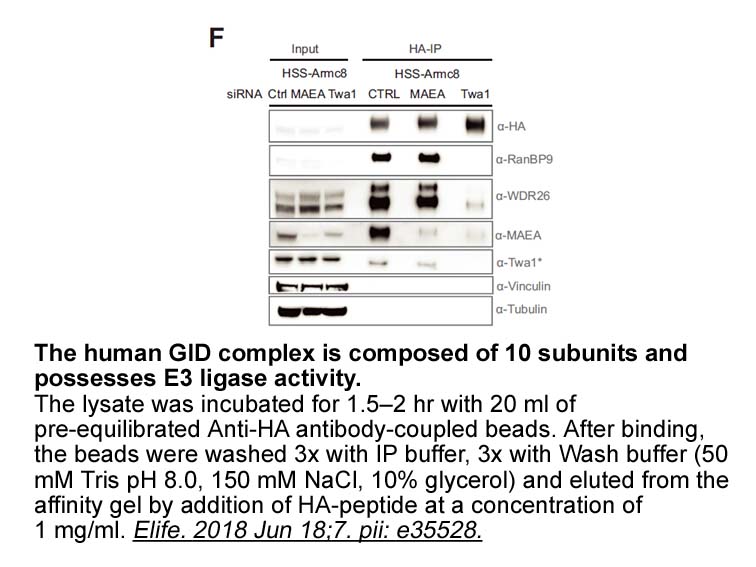
- 10. Lampert F, Stafa D, et al. "The multi-subunit GID/CTLH E3 ubiquitin ligase promotes cell proliferation and targets the transcription factor Hbp1 for degradation." Elife. 2018 Jun 18;7. pii: e35528. PMID: 29911972
| Storage | Desiccate at -20°C |
| M.Wt | 1102.15 |
| Cas No. | 92000-76-5 |
| Formula | C53H67N9O17 |
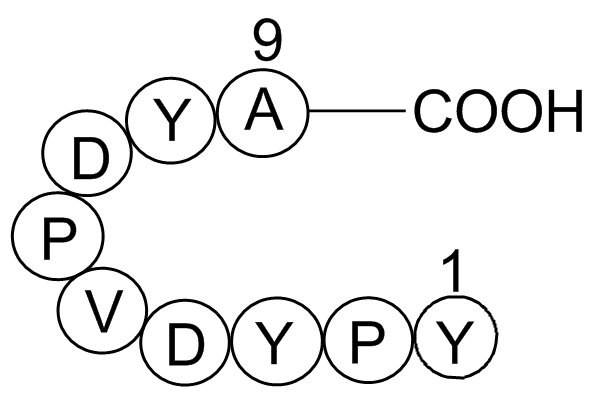
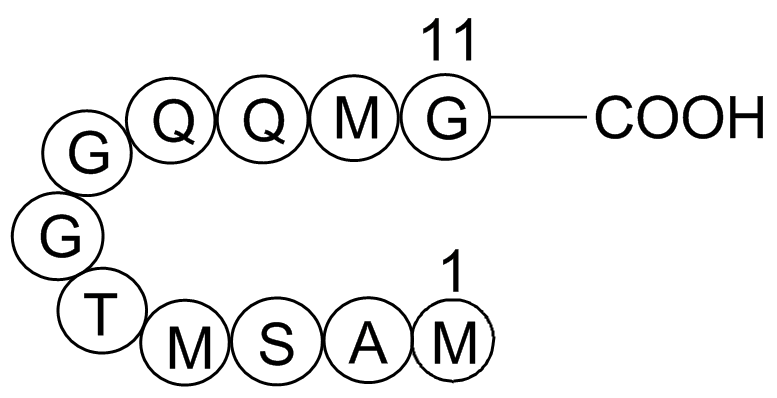
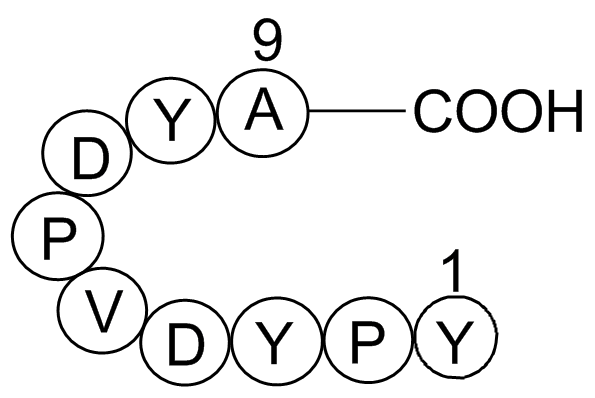
| Synonyms | H-Tyr-Pro-Tyr-Asp-Val-Pro-Asp-Tyr-Ala-OH |
| Solubility | ≥55.1 mg/mL in DMSO; ≥100.4 mg/mL in EtOH; ≥46.2 mg/mL in H2O |
| Chemical Name | Influenza Hemagglutinin (HA) Peptide |
| SDF | Download SDF |
| Canonical SMILES | CC(C)C(C(=O)N1CCCC1C(=O)NC(CC(=O)O)C(=O)NC(CC2=CC=C(C=C2)O)C(=O)NC(C)C(=O)O)NC(=O)C(CC(=O)O)NC(=O)C(CC3=CC=C(C=C3)O)NC(=O)C4CCCN4C(=O)C(CC5=CC=C(C=C5)O)N |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Description | Influenza Hemagglutinin (HA) Peptide is a tag peptide derived from an epitope of the influenza hemagglutinin protein. | |||||
| Targets | anti-HA-tag antibody | |||||
| IC50 | ||||||
Quality Control & MSDS
- View current batch:
Chemical structure
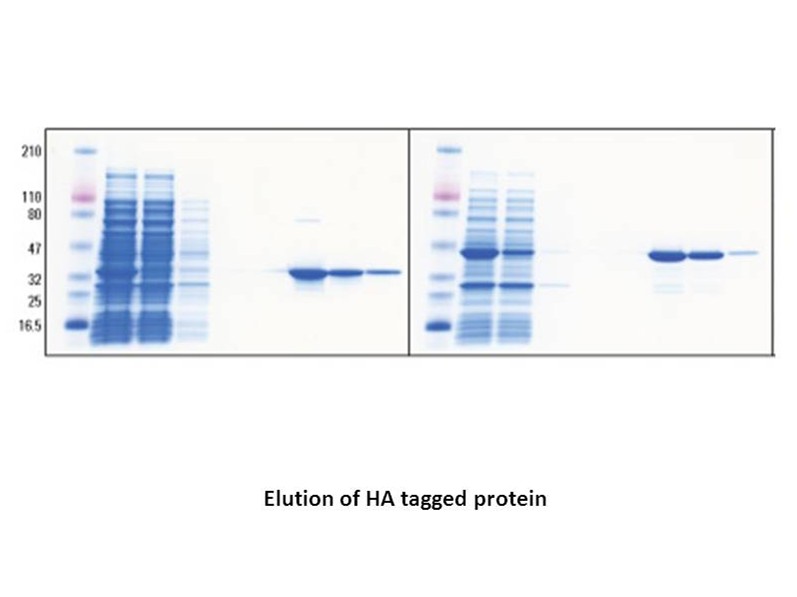
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data