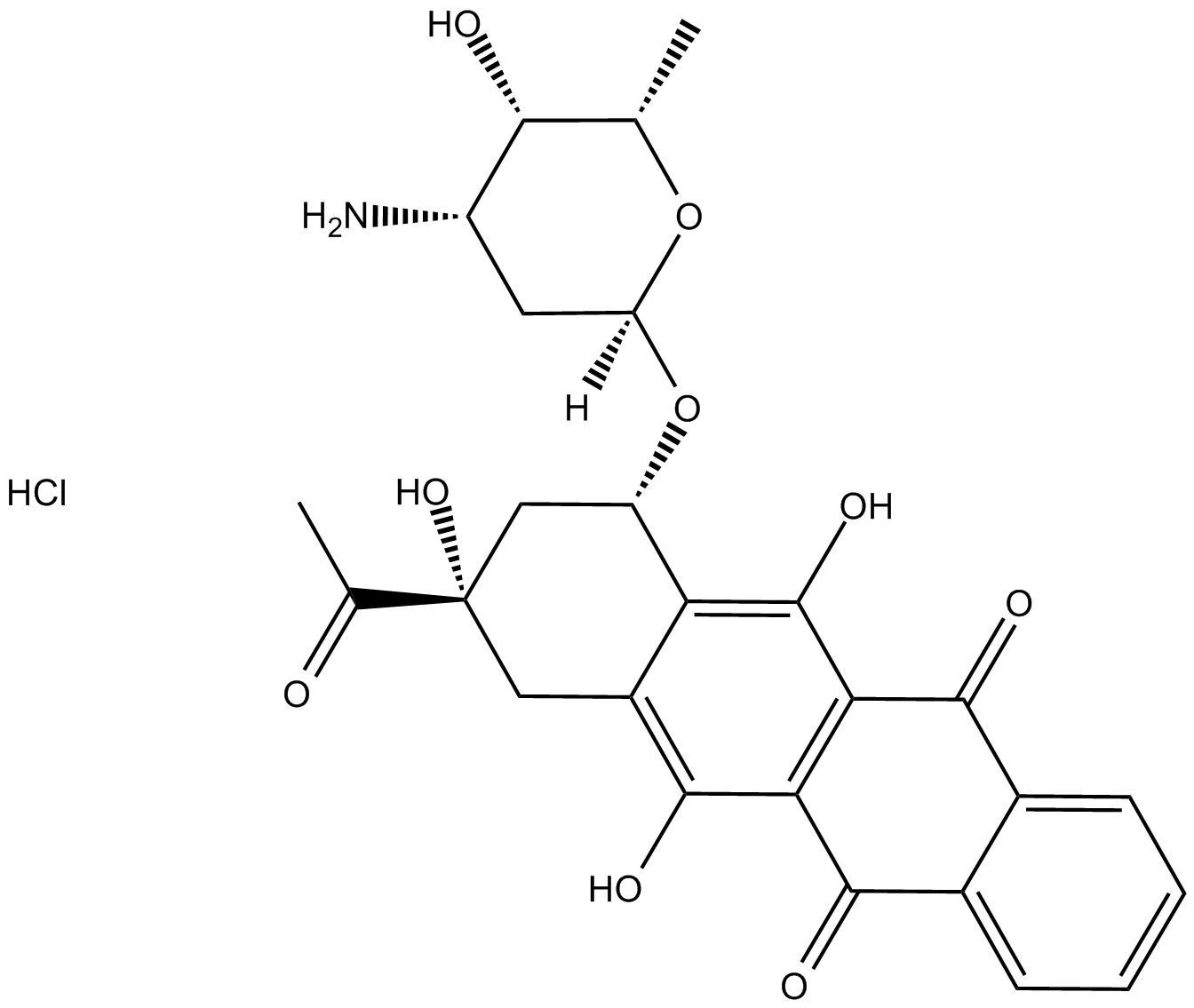
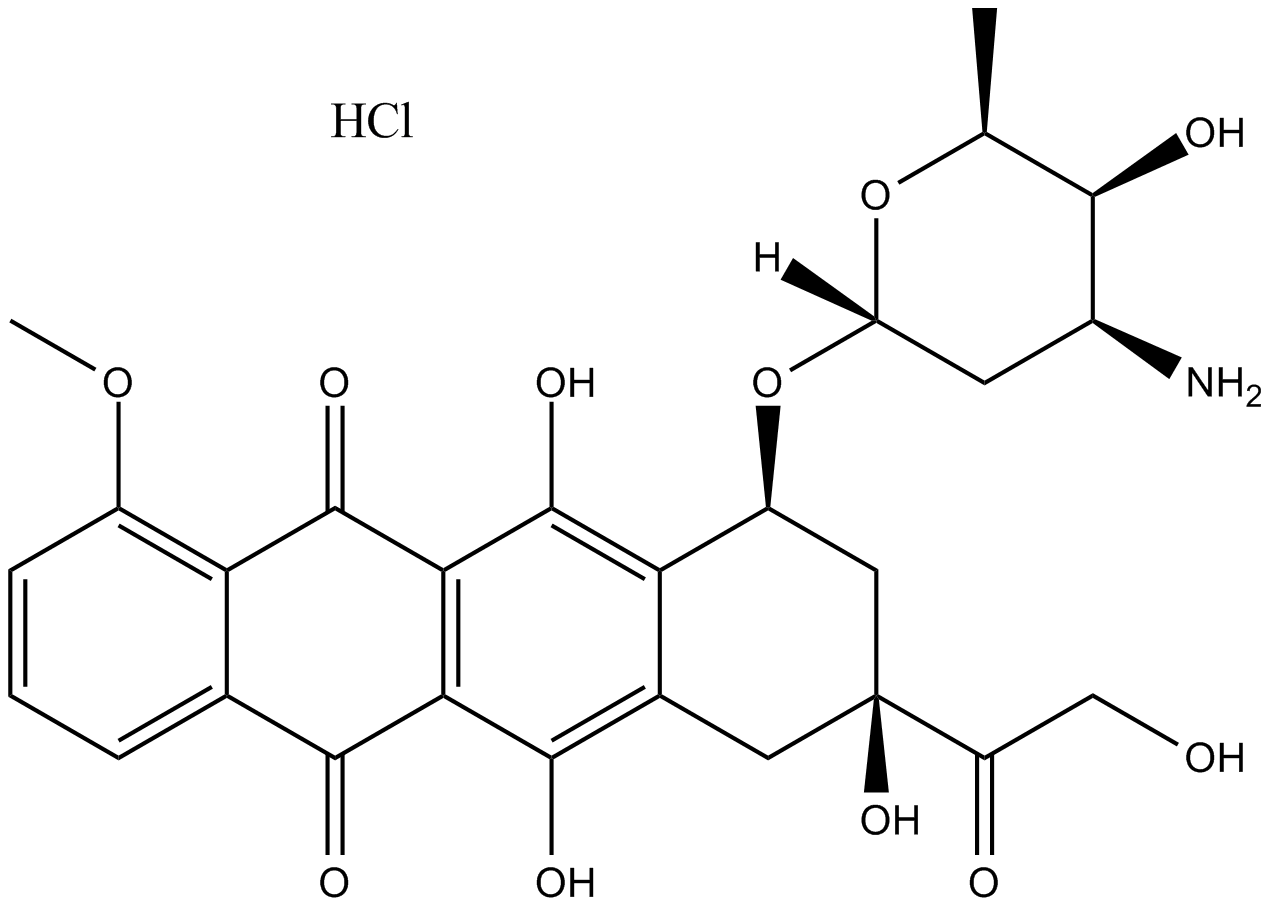
Idarubicin HCl
Idarubicin is an inhibitor of DNA topoisomerase II [1].
Idarubicin is a synthetic anthracycline anticancer drug widely used in the treatment of acute myelogenous leukemia and some other hematological malignancies. It can be bioactivated by NADPH-cytochrome P450 reductase with resulting formation of single-strand breaks in DNA. This is the mechanism of Idarubicin ‘s antitumor effect [2].
Idarubicin is developed in an attempt to reduce the cardiotoxicity and enhance the therapeutic efficacy of the parent compound. Unlike the parent compound, Idarubicin can be given orally and has a better therapeutic index with respect to cardiotoxicity. Idarubicin has been shown to be an effective anti-leukemic agent in children and adults [3].
References:
[1] H. Dorota Halicka, M. Fevzi Ozkaynak, Oya Levendoglu-Tugal, Claudio Sandoval , Karen Seiter, Malgorzata Kajstura, Frank Traganos, Somasunadaram Jayabose, and Zbigniew Darzynkiewicz. DNA damage response as a biomarker in treatment of leukemias. Cell Cycle. 2009, 8(11): 1720–1724.
[2] Haydar Çelik and Emel Arinç. Evaluation of the Protective Effects of Quercetin, Rutin, Resveratrol, Naringenin and Trolox Against Idarubicin-Induced DNA Damage. J Pharm Pharmaceut Sci. 2010, 13(2): 231 – 241.
[3] Ching-Hon Pui, Siebold S. N. de Graaf, Lois W. Dow, John H. Rodman, William E. Evans, Bruce S. Alpert and Sharon B. Murphy. Phase I Clinical Trial of Orally Administered 4-Demethoxydaunorubicin (Idarubicin) with Pharmacokinetic and in Vitro Drug Sensitivity Testing in Children with Refractory Leukemia. Cancer Research. 1988, 48: 5348-5352.
| Storage | Store at -20°C |
| M.Wt | 533.95 |
| Cas No. | 57852-57-0 |
| Formula | C26H28ClNO9 |
| Solubility | ≥26.7 mg/mL in DMSO; insoluble in EtOH; ≥2.39 mg/mL in H2O with ultrasonic |
| Chemical Name | (7S,9S)-9-acetyl-7-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | O=C(C1=C2C(O)=C3C([C@@H](O[C@]4([H])O[C@@H](C)[C@@H](O)[C@@H](N)C4)C[C@](O)(C(C)=O)C3)=C1O)C5=CC=CC=C5C2=O.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
NALM-6 cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reaction Conditions |
0.1 nM ~ 10 μM; 24 hrs |
|
Applications |
In NALM-6 cells, Idarubicin inhibited cell proliferation with an IC50 value of 12 nM. |
| Animal experiment [2]: | |
|
Animal models |
Rats, rabbits, mice and dogs |
|
Dosage form |
2 mg/kg, 0 mg/kg ~ 75 mg/kg, 3 mg/kg and 0 mg/kg ~ 75 mg/kg; i.v. |
|
Applications |
Reduction of Idarubicin was dependent upon ketone reductases, and proceeds more stereoselectivity than that of most ketones. The high stereospecificity in Idarubicin reduction might be attributed to chiral induction under the presence of asymmetric centres near to the carbonyl group in Idarubicin. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Rowland AJ, Pietersz GA, McKenzie IF. Preclinical investigation of the antitumour effects of anti-CD19-idarubicin immunoconjugates. Cancer Immunol Immunother. 1993 Aug;37(3):195-202. [2]. Strolin Benedetti M, Pianezzola E, Fraier D, Castelli MG, Dostert P. Stereoselectivity of idarubicin reduction in various animal species and humans. Xenobiotica. 1991 Apr;21(4):473-80. |
|
Quality Control & MSDS
- View current batch:
Chemical structure