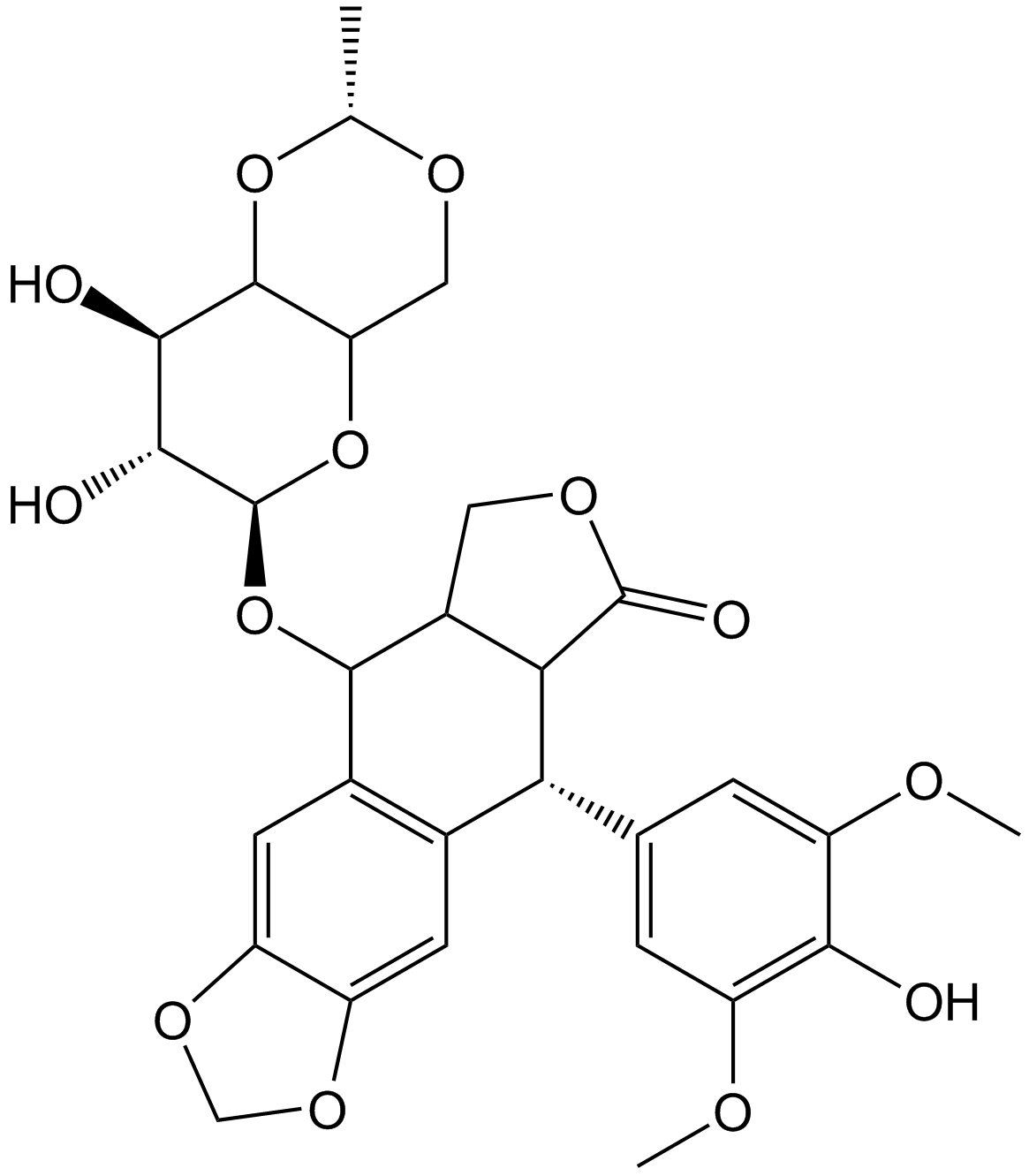
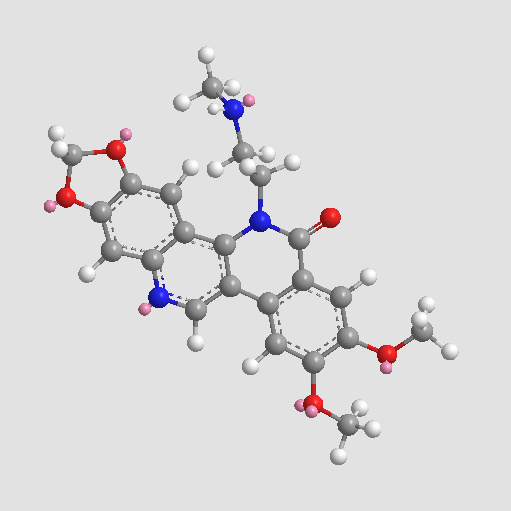
Etoposide
Etoposide (VP-16) is the first agent recognized as a topoisomerase II inhibitor of anticancer drug with IC50 of 59.2 μM.
The activity of the topoisomerase II enzyme on re-ligation of DNA strands is interrupted by etoposide. A ternary complex with DNA is formed by etoposide, and causes DNA strands to break [1]. The enzyme was more important in cancer cell than healthy cells, because cancer cells divided more rapidly. So etoposide induced apoptosis of the cancer cells [2]. Etoposide exhibited cytotoxic activity against HepG2 and MOLT-3 cancer cells with IC50 of 30.16 μM and 0.051μM [3]. The IC50 values of etoposide against the tumor cell lines of BGC-823, HeLa, and A549 were 43.74 ± 5.13, 209.90 ± 13.42, and 139.54 ± 7.05 μM, respectively [4].
References:
[1] Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010 May 28; 17 (5): 421-33.
[2] Gordaliza M, García PA, del Corral JM, Castro MA, Gómez-Zurita MA. Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives. Toxicon. 2004 Sep 15; 44 (4): 441-59.
[3] Pingaew R, Mandi P, Nantasenamat C, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. Design, synthesis and molecular docking studies of novel N-benzenesulfonyl-1,2,3,4-tetrahydroisoquinoline-based triazoles with potential anticancer activity. Eur J Med Chem. 2014 May 6; 81C: 192-203.
[4] Xiao L, Zhao W, Li HM, Wan DJ, Li DS, Chen T, Tang YJ. Design and synthesis of the novel DNA topoisomerase II inhibitors: Esterification and amination substituted 4'-demethylepipodophyllotoxin derivates exhibiting anti-tumor activity by activating ATM/ATR signaling pathways. Eur J Med Chem. 2014 Jun 10; 80: 267-77.
- 1. Lucy Martin, Anna Irving, et al. "Machine learning recognises senescence in glioblastoma and discovers senescence-inducing compounds." bioRxiv. April 04, 2024.
- 2. Zhengyi Zhen, Yu Chen, et al. "Nuclear cGAS restricts L1 retrotransposition by promoting TRIM41-mediated ORF2p ubiquitination and degradation." Nat Commun. 2023 Dec 12;14(1):8217. PMID: 38086852
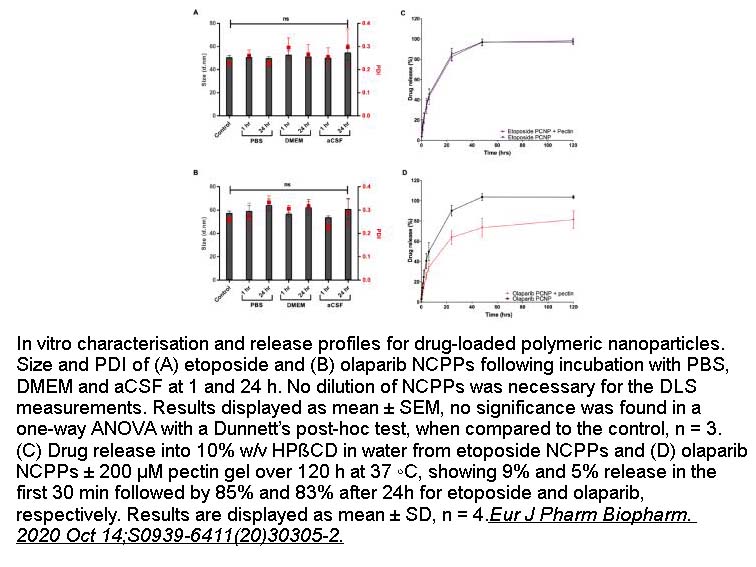
- 3. Phoebe McCrorie, Jatin Mistry, et al. "Etoposide and olaparib polymer-coated nanoparticles within a bioadhesive sprayable hydrogel for post-surgical localised delivery to brain tumours." Eur J Pharm Biopharm. 2020 Oct 14;S0939-6411(20)30305-2. PMID:33068736
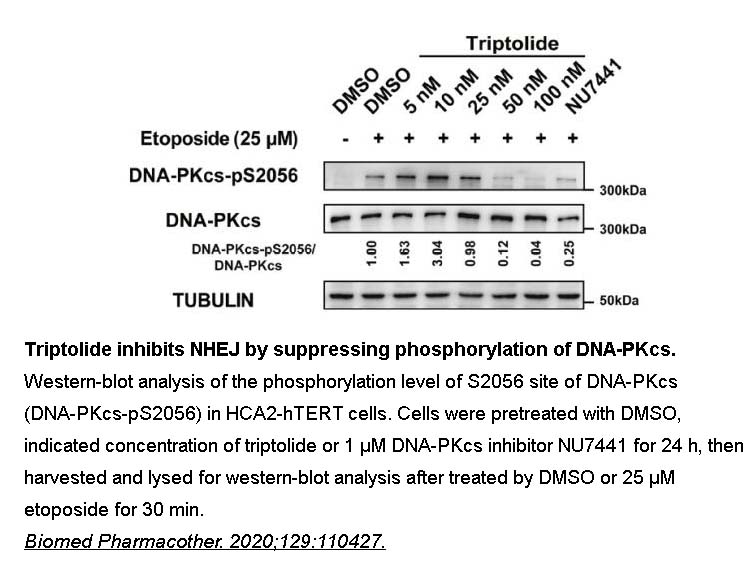
- 4. Cai B, Hu Z, et al. "Triptolide impairs genome integrity by directly blocking the enzymatic activity of DNA-PKcs in human cells." Biomed Pharmacother. 2020;129:110427. PMID:32574974
- 5. Zhao K, Wang X, et al. "A long noncoding RNA sensitizes genotoxic treatment by attenuating ATM activation and homologous recombination repair in cancers." PLoS Biol. 2020;18(3):e3000666. PMID:32203529
- 6. Wang Z, Chen J, et al. "cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity." J Clin Invest. 2019 Aug 13;130:4850-4862. PMID:31408442
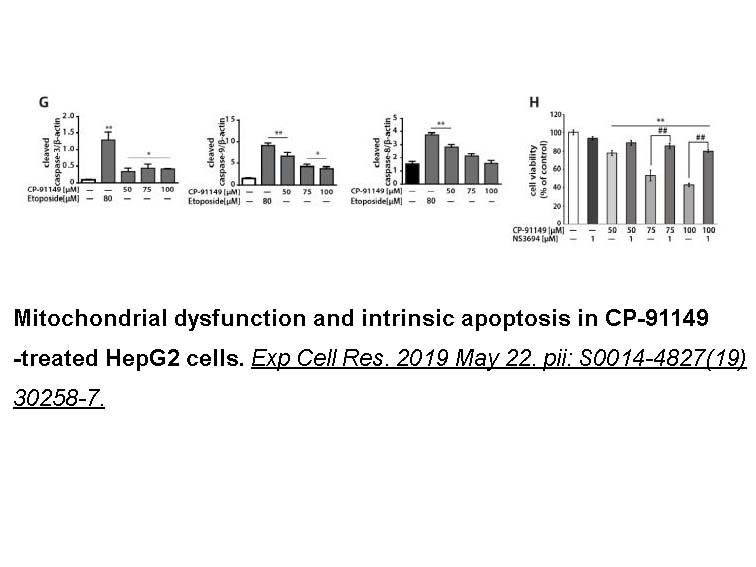
- 7. Barot S, Abo-Ali EM, et al. "Inhibition of glycogen catabolism induces intrinsic apoptosis and augments multikinase inhibitors in hepatocellular carcinoma cells." Exp Cell Res. 2019 Aug 15;381(2):288-300. PMID:31128107
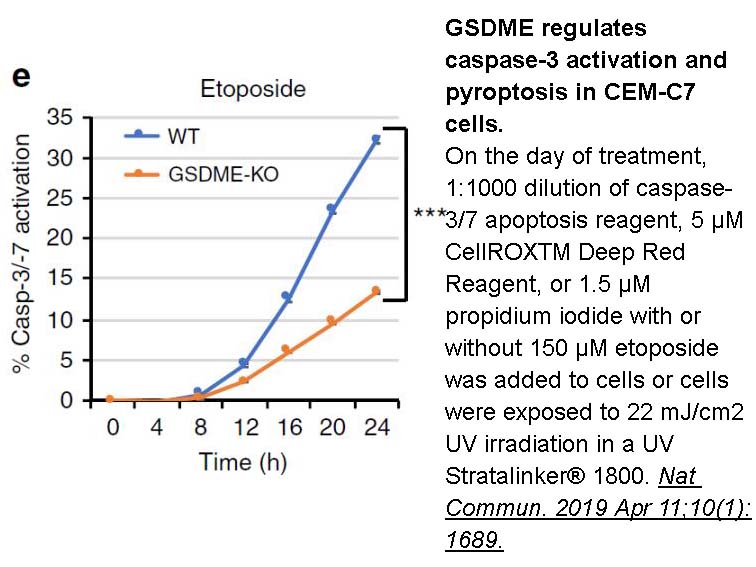
- 8. Rogers C, Erkes DA, et al. "Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation." Nat Commun. 2019 Apr 11;10(1):1689. PMID:30976076
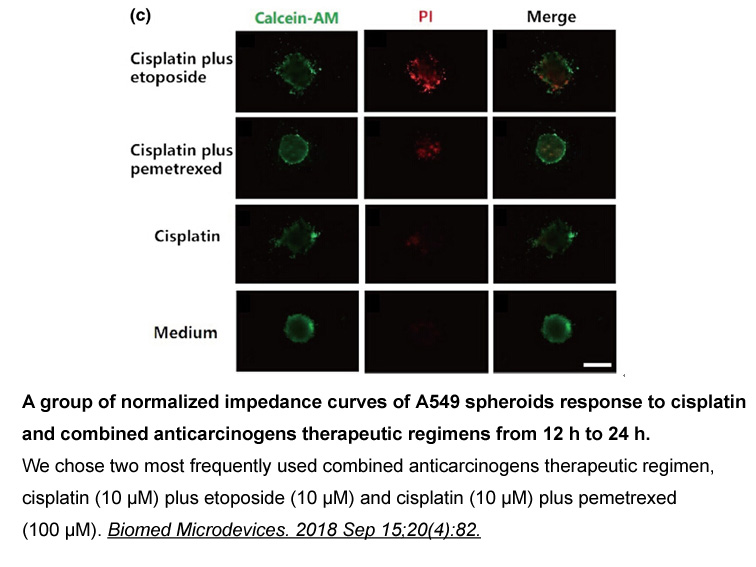
- 9. Wu Q, Wei X, et al. "Bionic 3D spheroids biosensor chips for high-throughput and dynamic drug screening." Biomed Microdevices. 2018 Sep 15;20(4):82. PMID:30220069
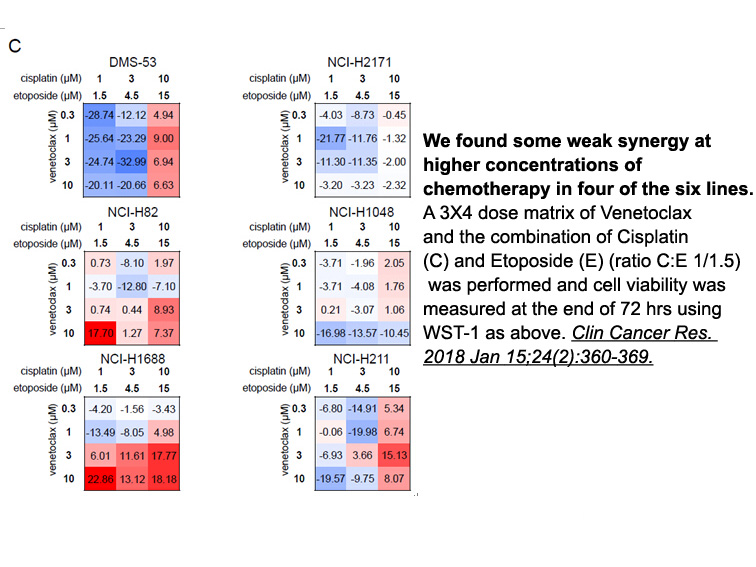
- 10. Lochmann TL, Floros KV, et al. "Venetoclax Is Effective in Small-Cell Lung Cancers with High BCL-2 Expression." Clin Cancer Res. 2018 Jan 15;24(2):360-369. PMID:29118061
- 11. Xia L, Xiao X, et al. "Coactosin-like protein CLP/Cotl1 suppresses breast cancer growth through activation of IL-24/PERP and inhibition of non-canonical TGFβ signaling." Oncogene. 2018 Jan 18;37(3):323-331. PMID:28925397
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 588.56 |
| Cas No. | 33419-42-0 |
| Formula | C29H32O13 |
| Solubility | ≥112.6 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
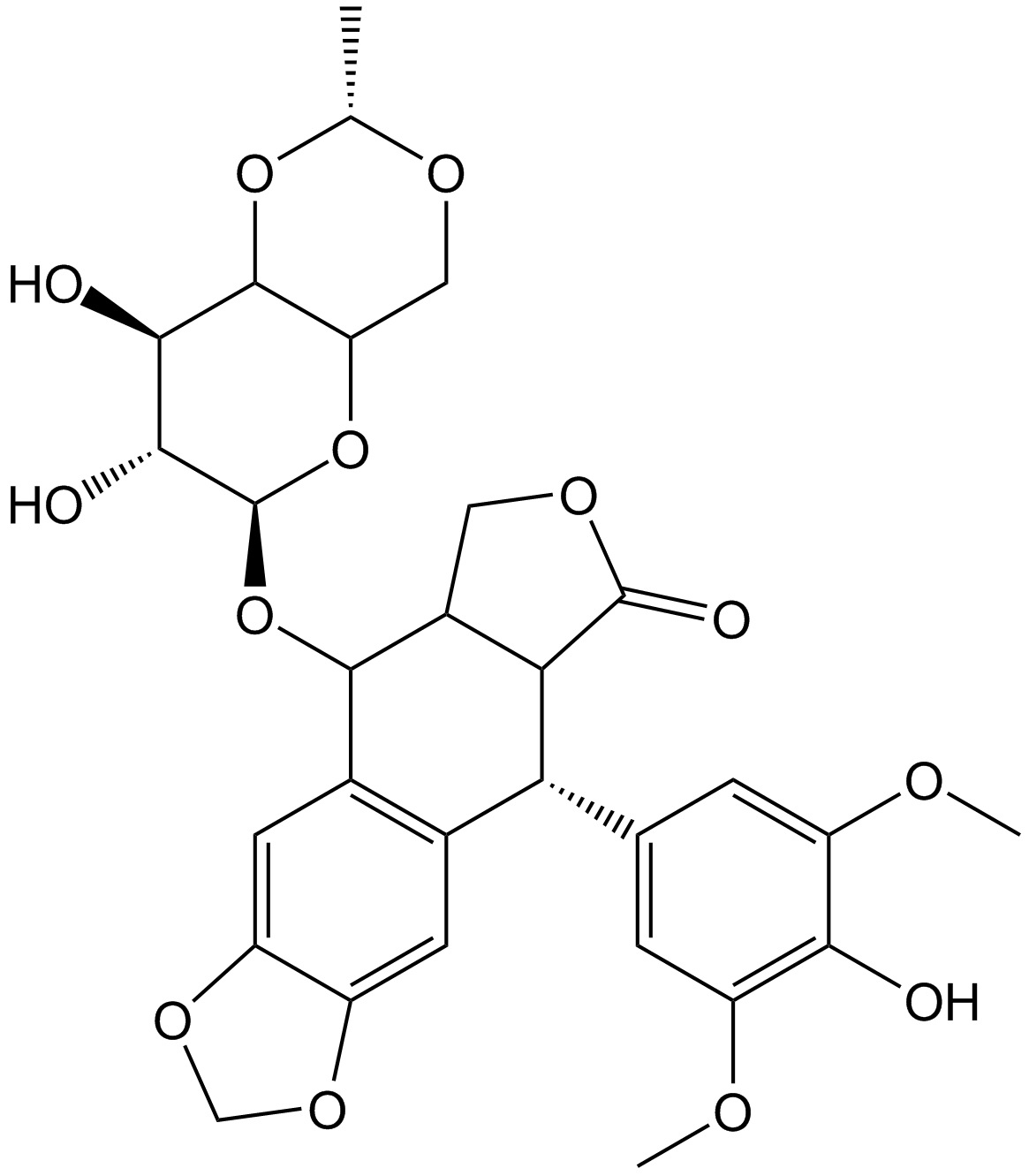
| Chemical Name | (5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one |
| SDF | Download SDF |
| Canonical SMILES | CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Topoisomerase II activity assay |
Nuclear extracts are prepared, and nuclei are isolated. The activity of topoisomerase II is calculated from the percentage of decatenation obtained. Tritiated kinoplast DNA (KDNA 0.22 μg) is used as a substrate. Etoposide and topoisomerase II are incubated for 30 min at 37°C and are stopped with 1% sodium dodecyl sulfate (SDS) and proteinase K (100 μg/mL). The percentages of decatenation and inhibition of topoisomerase II by Etoposide are obtained. |
| Cell experiment [2]: | |
|
Cell lines |
BGC-823, HeLa and A549 cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
0.5, 1, 10, 20, 50, 100, 200 and 500 μM; 2 d |
|
Applications |
The IC50 values of Etoposide against the tumor cell lines of BGC-823, HeLa and A549 were 43.74 ± 5.13 μM, 209.90 ± 13.42 μM and 139.54 ± 7.05 μM, respectively. |
| Animal experiment [3]: | |
|
Animal models |
Murine angiosarcoma xenografts ISOS-1 |
|
Dosage form |
2.5, 5, 10 mg/kg; i.p.; every day for 5 days |
|
Applications |
The dose of 10 mg/kg Etoposide (i.p.) inhibited murine angiosarcoma cell ISOS-1 tumors. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Beauchesne P, Bertrand S, N'guyen MJ, Christianson T, Dore JF, Mornex F, Bonner JA. Etoposide sensitivity of radioresistant human glioma cell lines. Cancer Chemother Pharmacol. 1998; 41 (2): 93-7. [2] Xiao L, Zhao W, Li HM, Wan DJ, Li DS, Chen T, Tang YJ. Design and synthesis of the novel DNA topoisomerase II inhibitors: Esterification and amination substituted 4'-demethylepipodophyllotoxin derivates exhibiting anti-tumor activity by activating ATM/ATR signaling pathways. Eur J Med Chem. 2014 Jun 10; 80: 267-77. [3] Ma G1, Masuzawa M, Hamada Y, Haraguchi F, Tamauchi H, Sakurai Y, Fujimura T, Katsuoka K. Treatment of murine angiosarcoma with etoposide, TNP-470 and prednisolone. J Dermatol Sci. 2000 Nov; 24 (2): 126-33. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data