Voreloxin
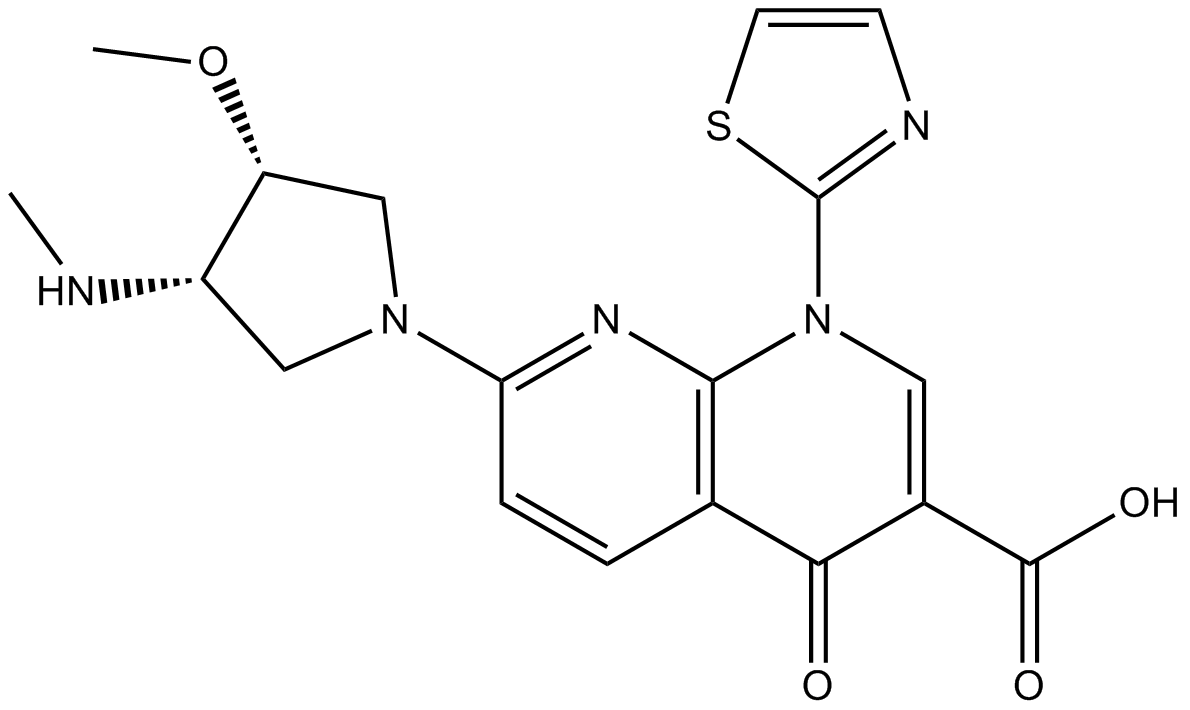
Voreloxin (CAS: 175414-77-4), also known as SNS-595 or AG-7352, is a novel naphthyridine derivative structurally related to quinolone antibiotics, a class previously unused in oncology. Voreloxin exerts its antitumor activity by intercalating DNA and inhibiting topoisomerase II, leading to DNA damage and apoptosis. In vitro, voreloxin demonstrates potent antiproliferative effects across diverse tumor cell lines, with IC50 values between 0.04 and 0.97 μM, including lines overexpressing P-glycoprotein. In vivo, it inhibits tumor growth in xenograft models in a dose-dependent manner. Voreloxin is under investigation for its potential in treating ovarian cancer and acute myeloid leukemia.
References:
[1] Tsuzuki Y, Tomita K, Shibamori K, Sato Y, Kashimoto S, Chiba K. Synthesis and structure-activity relationships of novel 7-substituted 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acids as antitumor agents. Part 2. J Med Chem.2004;47(8):2097-109.
[2] Hoch U, Lynch J, Sato Y, Kashimoto S, Kajikawa F, Furutani Y, Silverman JA. Voreloxin, formerly SNS-595, has potent activity against a broad panel of cancer cell lines and in vivo tumor models. Cancer Chemother Pharmacol. 2009;64(1):53-65.
[3] Advani RH, Hurwitz HI, Gordon MS, Ebbinghaus SW, Mendelson DS, Wakelee HA, Hoch U, Silverman JA, Havrilla NA, Berman CJ, Fox JA, Allen RS, Adelman DC. Voreloxin, a first-in-class anticancer quinolone derivative, in relapsed/refractory solid tumors: a report on two dosing schedules. Clin Cancer Res. 2010;16(7):2167-75.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 401.44 |
| Cas No. | 175414-77-4 |
| Formula | C18H19N5O4S |
| Synonyms | SNS-595; Vosaroxin; AG 7352 |
| Solubility | insoluble in H2O; insoluble in EtOH; insoluble in DMSO |
| Chemical Name | 7-[(3S,4S)-3-methoxy-4-(methylamino)pyrrolidin-1-yl]-4-oxo-1-(1,3-thiazol-2-yl)-1,8-naphthyridine-3-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | CN[C@@H](CN(C1)c2ccc(C(C(C(O)=O)=CN3c4ncc[s]4)=O)c3n2)[C@H]1OC |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
SK-BR-3, ScaBER, PANC-1, KB, HCT116, SKOV3, GT3TKB, Hs746T, Calu-6, NCI-H460, PA-1, MES-SA, SBC-3, SBC-3/ETP and PC-14 cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reaction Conditions |
0.04 ~ 1.155 μM; 72 hrs |
|
Applications |
Voreloxin exhibited broad anti-proliferative activity in 15 cell lines, including 4 drug-resistant lines, with the IC50 values ranging from 0.04 to 1.155 μM. |
| Animal experiment [2]: | |
|
Animal models |
Mice implanted with P388 leukemia cells |
|
Dosage form |
3.13, 12.5 or 50 mg/kg; i.p.; on days 1 and 5 after tumor implantation |
|
Applications |
In mice implanted with P388 leukemia cells, Voreloxin (50 mg/kg, i.p.) showed potent antitumor activity. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Hoch U, Lynch J, Sato Y, Kashimoto S, Kajikawa F, Furutani Y, Silverman JA. Voreloxin, formerly SNS-595, has potent activity against a broad panel of cancer cell lines and in vivo tumor models. Cancer Chemother Pharmacol. 2009;64(1):53-65. [2]. Tsuzuki Y, Tomita K, Shibamori K, Sato Y, Kashimoto S, Chiba K. Synthesis and structure-activity relationships of novel 7-substituted 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acids as antitumor agents. Part 2. J Med Chem. 2004;47(8):2097-109. | |
| Description | Voreloxin is an inhibitor of topoisomerase II. | |||||
| Targets | topoisomerase II | |||||
| IC50 | ||||||
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure