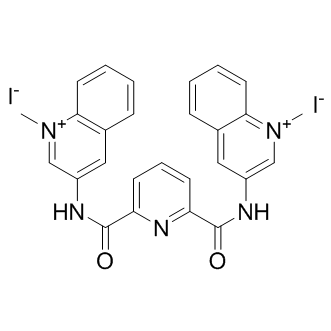
360A iodide
Description: IC50 Value: N/A 360A is a 2,6-pyridine-dicarboxamide derivative displaying strong affinity and selectivity for G-quadruplex structures and selective telomerase inhibition in vitro assays. 360A is a G-quadruplex ligand, which can influence the consequence of G-quadruplex formation and/or stabilization. in vitro: We found a S-phase accumulation in ATM-proficient, but not in ATM-deficient EBV-lymphocytes treated with 360A before induction of cell death. However, ATM status did not modify cell cycle distribution in 360A-treated SV40-fibroblasts and HeLa cells compared to DMSO treated controls [1]. DNA-PKcs-dependent NHEJ was responsible for sister telomere fusions as a direct consequence of G-quadruplex formation and/or stabilization induced by 360A on parental telomere G strands. NHEJ and HR activation at telomeres altered mitotic progression in treated cells [2]. This compound was shown to display a potent affinity and selectivity for telomeric G-quadruplex DNA over duplex DNA and to induce delayed growth inhibition in HT1080 tumor cell line [3]. in vivo: N/A Toxicity: N/A Clinical trial: N/A
| Storage | Store at -20°C |
| M.Wt | 703.31 |
| Cas No. | 737763-37-0 |
| Formula | C27H23I2N5O2 |
| Synonyms | 360 A iodide |
| Solubility | insoluble in H2O; insoluble in EtOH; insoluble in DMSO |
| Chemical Name | (3Z,3'Z)-3,3'-((pyridine-2,6-dicarbonyl)bis(azanylylidene))bis(1-methyl-2,3-dihydroquinolin-1-ium-2-ide) dihydroiodide |
| SDF | Download SDF |
| Canonical SMILES | CN1=C2C=CC=CC2=C/C(C=1)=N/C(C3=NC(C(/N=C4C=C5C=CC=CC5=N(C)=C/4)=O)=CC=C3)=O.I.I |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure