Pyridostatin
Pyridostatin (CAS 1085412-37-8) is a synthetic small molecule functioning as a stabilizer of G-quadruplex DNA structures. G-quadruplexes are secondary DNA structures enriched in guanine-rich regions, such as telomeres and promoter sequences of certain oncogenes (e.g., c-kit, K-ras, Bcl-2), affecting DNA replication, transcription regulation, and telomere integrity. Pyridostatin selectively binds and stabilizes these structures, leading to competitive inhibition of telomere-associated protein binding and subsequent induction of telomere dysfunction. In cellular assays, Pyridostatin selectively inhibits growth of various cancer cell lines (HeLa, U2OS, HT1080) with IC50 values ranging from 0.89 to 10 μM, demonstrating potential utility for cancer biology research.
References:
[1] Mela I, Kranaster R, Henderson R M, et al. Demonstration of ligand decoration, and ligand-induced perturbation, of G-quadruplexes in a plasmid using atomic force microscopy. Biochemistry, 2012, 51(2): 578-585.
[2] Müller S, Sanders D A, Di Antonio M, et al. Pyridostatin analogues promote telomere dysfunction and long-term growth inhibition in human cancer cells. Organic & biomolecular chemistry, 2012, 10(32): 6537-6546.
[3] McLuckie K I E, Di Antonio M, Zecchini H, et al. G-quadruplex DNA as a molecular target for induced synthetic lethality in cancer cells. Journal of the American Chemical Society, 2013, 135(26): 9640-9643.
- 1. Zhenzhen Yan, Axin He, et al. "Structural Insights into an Antiparallel Chair‐Type G‐Quadruplex From the Intron of NOP56 Oncogene." Adv Sci (Weinh). 2025 Apr;12(16):e2406230 PMID: 40047221
- 2. Emily G. Oldani, Kevin M. Reynolds Caicedo, et al. "Manipulating TDP43 Aggregation via RNA G-quadruplexes." bioRxiv. April 30, 2024.
- 3. Sowers ML, Conrad JW, et al. "DNA Base Excision Repair Intermediates Influence Duplex–Quadruplex Equilibrium." Molecules 2023 Jan 18;28(3) PMID: 36770637
- 4. Vivek M Shastri, Veena Subramanian, et al. "A novel cell-cycle-regulated interaction of the Bloom syndrome helicase BLM with Mcm6 controls replication-linked processes." Nucleic Acids Res. 2021 Sep 7;49(15):8699-8713. PMID: 34370039
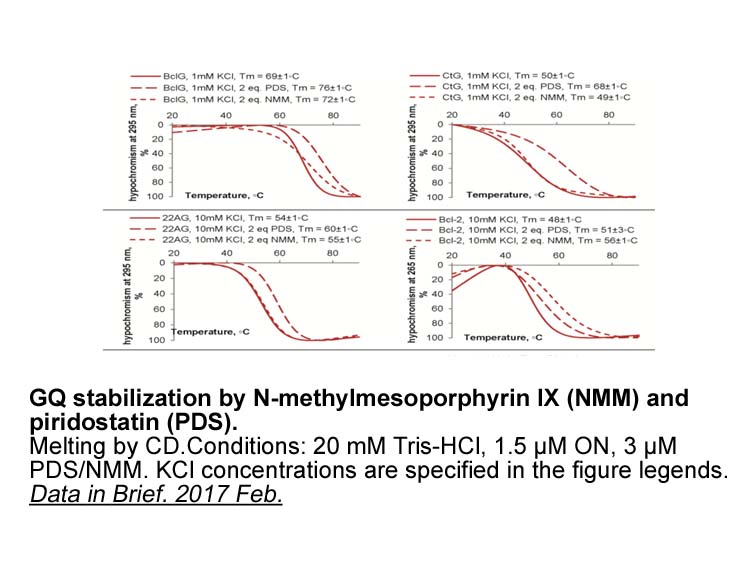
- 5. Vlasenok M, Varizhuk A, et al. "Data on secondary structures and ligand interactions of G-rich oligonucleotides that defy the classical formula for G4 motifs." Data Brief. 2017 Feb 12;11:258-265. PMID: 28243622
- 6. Varizhuk A, Ischenko D, et al."The expanding repertoire of G4 DNA structures." Biochimie. 2017 Apr;135:54-62. PMID: 28109719
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 596.64 |
| Cas No. | 1085412-37-8 |
| Formula | C31H32N8O5 |
| Synonyms | RR-82;RR82;RR 82 |
| Solubility | ≥20.85 mg/mL in DMSO; ≥30.87 mg/mL in EtOH with gentle warming; ≥9.66 mg/mL in H2O with gentle warming and ultrasonic |
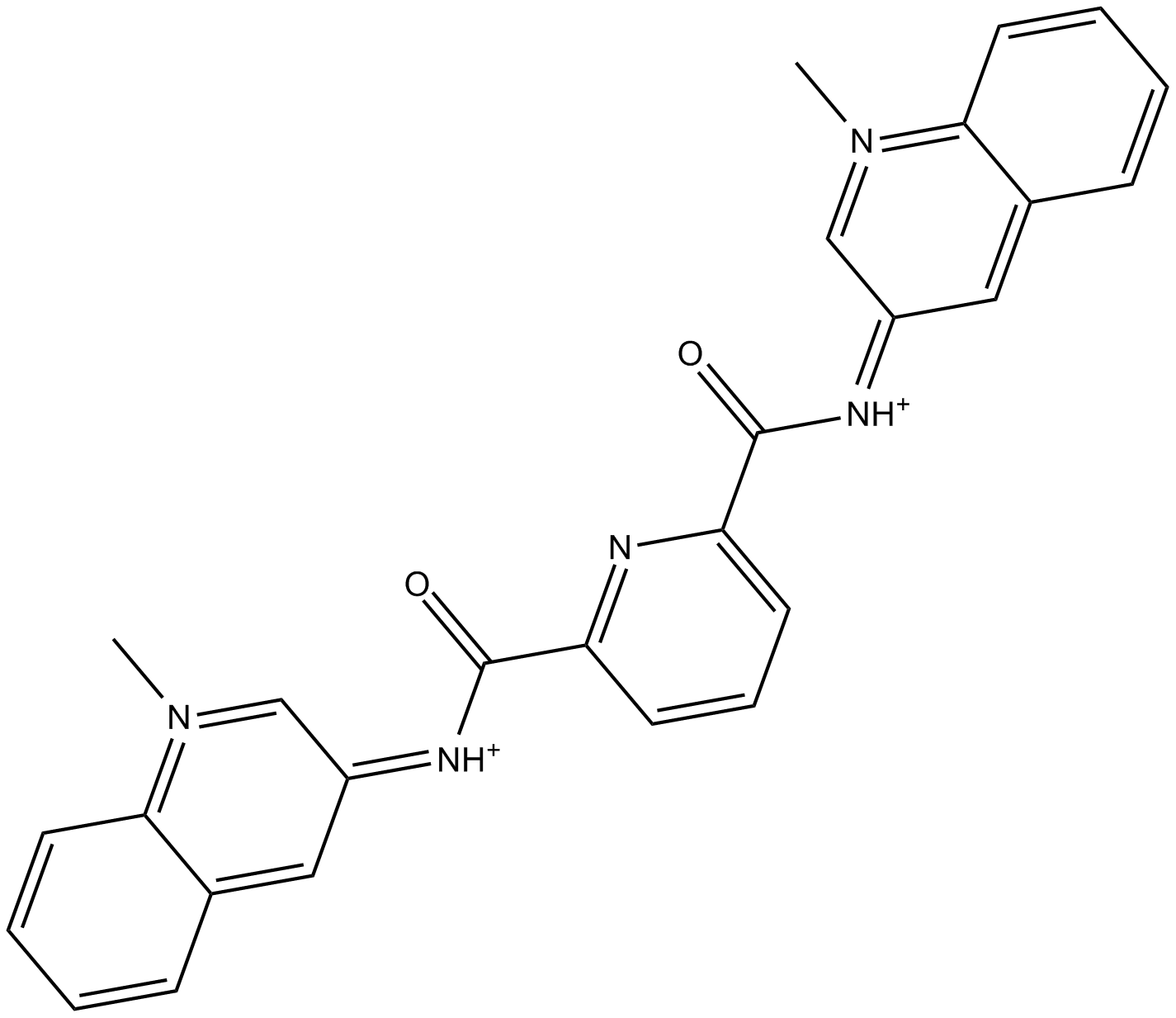
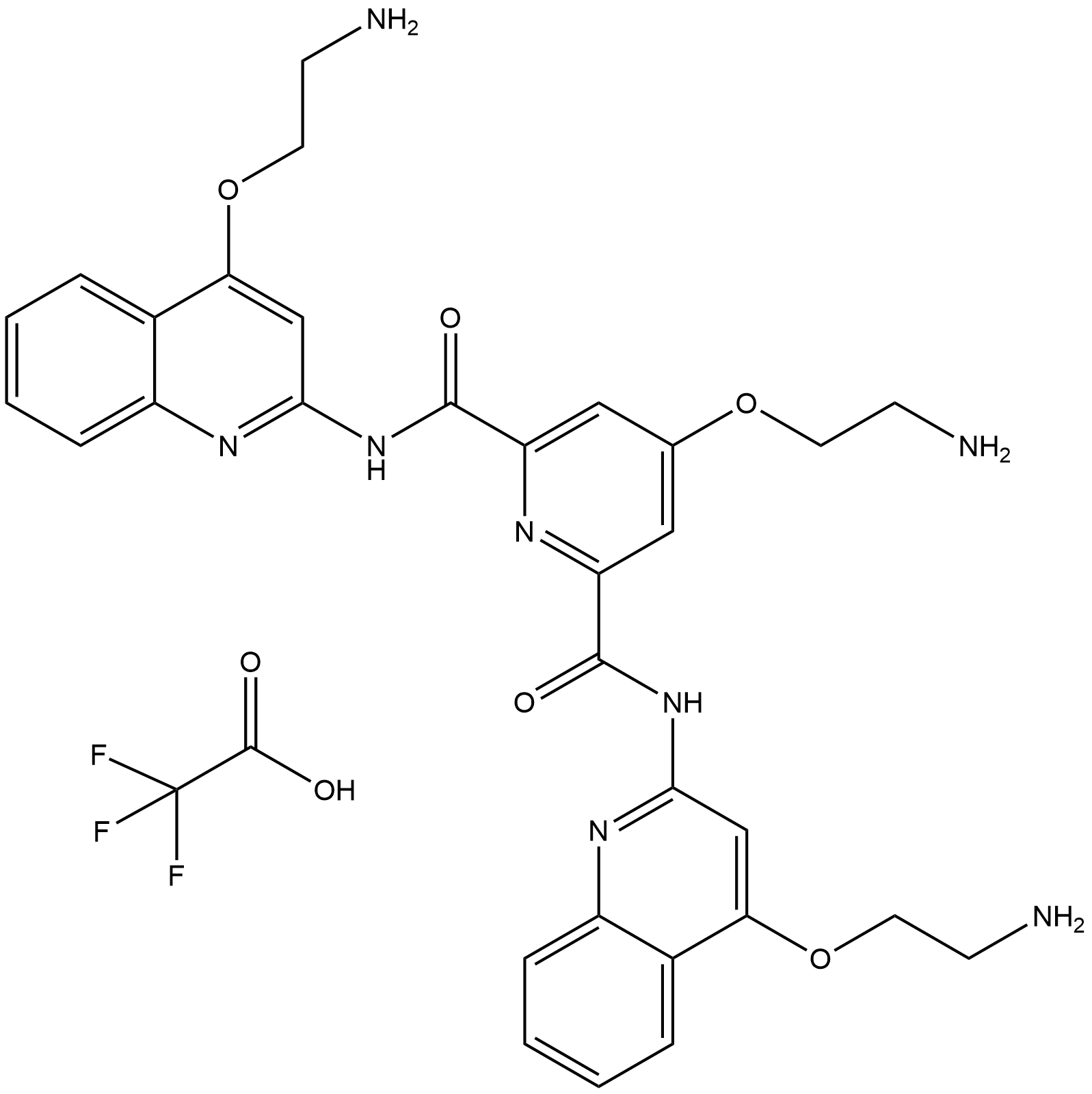
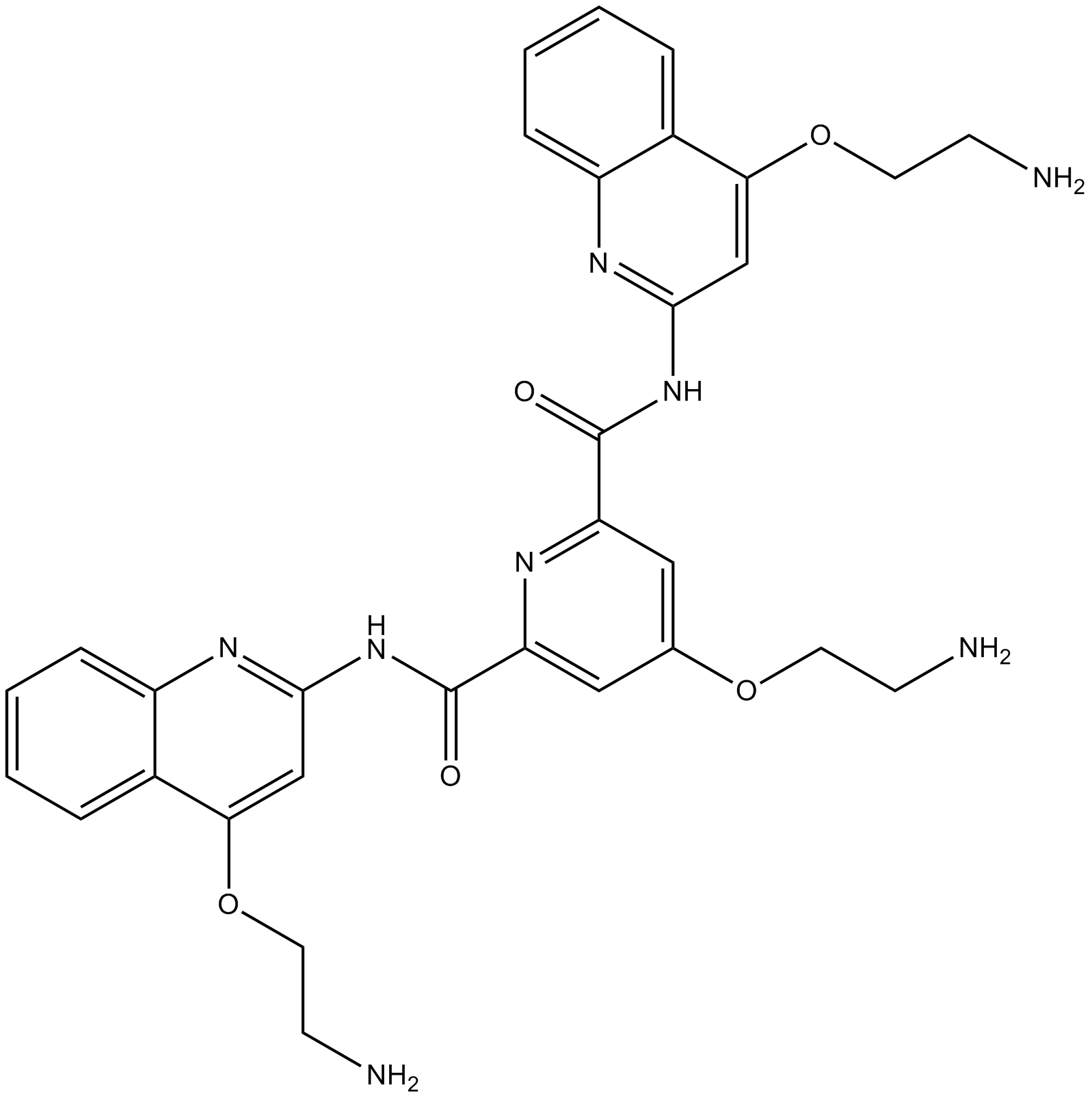
| Chemical Name | 4-(2-aminoethoxy)-2-N,6-N-bis[4-(2-aminoethoxy)quinolin-2-yl]pyridine-2,6-dicarboxamide |
| SDF | Download SDF |
| Canonical SMILES | C1=CC=C2C(=C1)C(=CC(=N2)NC(=O)C3=CC(=CC(=N3)C(=O)NC4=NC5=CC=CC=C5C(=C4)OCCN)OCCN)OCCN |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
HeLa, HT1080, U2OS and WI-38 cell lines |
|
Preparation method |
The solubility of this compound in DMSO is >20.85 mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0–40 μM for 72 h |
|
Applications |
A previous study investigated the growth inhibition after 3 days of exposure to pyridostatin on a panel of four human cell lines: HeLa (adenocarcinoma), HT1080 (fibrosarcoma), U2OS (osteosarcoma), and WI-38 (normal lung fibroblasts), the latter being non-cancerous. Pyridostatin showed growth inhibition at high nanomolar to low micromolar concentrations against these tested cell lines. In addition, pyridostatin exhibited an 18.5-fold selectivity for HT1080 cells over WI-38 cells. |
|
References: [1] Müller S, Sanders D A, Di Antonio M, et al. Pyridostatin analogues promote telomere dysfunction and long-term growth inhibition in human cancer cells. Organic & biomolecular chemistry, 2012, 10(32): 6537-6546. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data