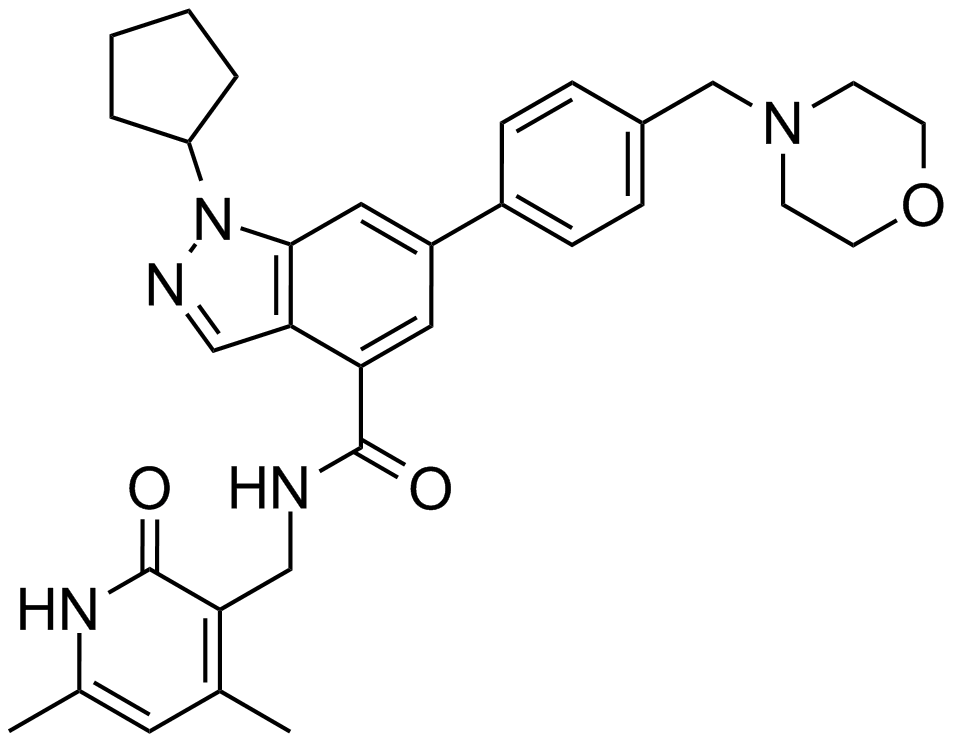
UNC1999
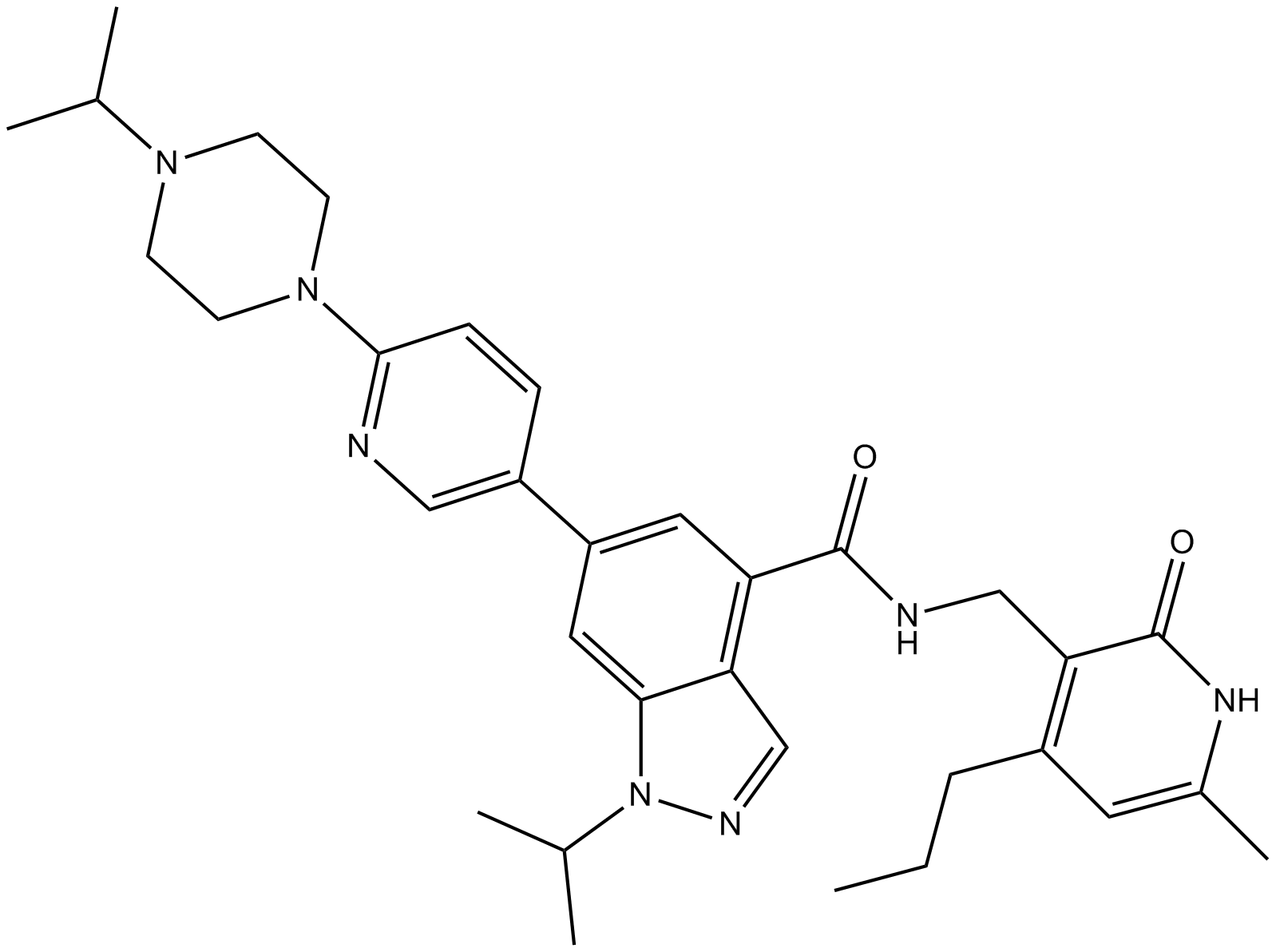
UNC1999 (CAS: 1431612-23-5) is a small molecule inhibitor targeting EZH2 and EZH1, enzymes responsible for methylating lysine 27 on histone H3 (H3K27me3), a modification associated with transcriptional suppression. It inhibits EZH2 and EZH1 with IC50 values of 2 nM and 45 nM, respectively, binding at the enzyme's SAM cofactor binding site but not competing with histone substrate. UNC1999 reduces cellular H3K27me3 levels (IC50 = 124 nM) and inhibits proliferation of DLBCL cells harboring EZH2 Y641N mutation (EC50 = 633 nM). It exhibits oral bioavailability in mouse studies, supporting its utility as a research probe for investigating EZH-mediated epigenetic regulation.
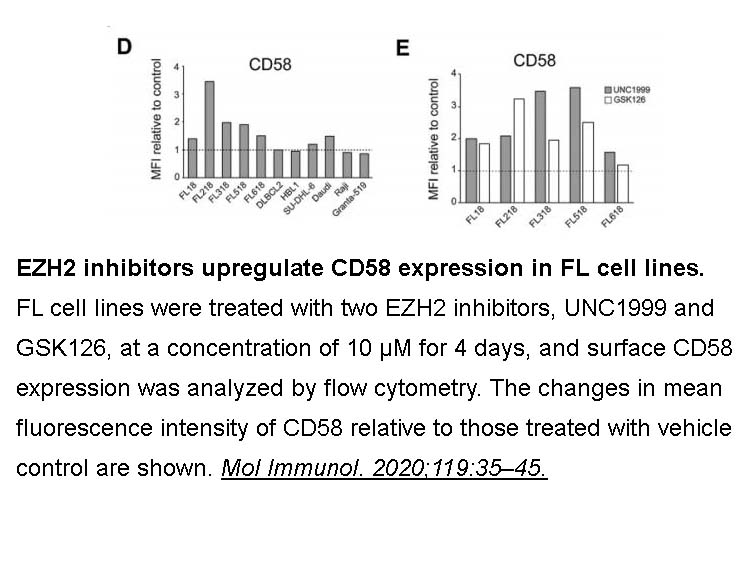
- 1. Otsuka Y, Nishikori M, et al. "EZH2 inhibitors restore epigenetically silenced CD58 expression in B-cell lymphomas." Mol Immunol. 2020;119:35–45 PMID: 31962268
- 2. Li N, Yang L, et al. "BET bromodomain inhibitor JQ1 preferentially suppresses EBV-positive nasopharyngeal carcinoma cells partially through repressing c-Myc." Cell Death Dis. 2018 Jul 9;9(7):761 PMID: 29988031
- 3. Lin B, Coleman JH, et al. "Injury Induces Endogenous Reprogramming and Dedifferentiation of NeuronalProgenitors to Multipotency." Cell Stem Cell. 2017 Nov 20. pii:S1934-5909(17)30375-2 PMID: 29174332
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 569.74 |
| Cas No. | 1431612-23-5 |
| Formula | C33H43N7O2 |
| Solubility | insoluble in H2O; insoluble in EtOH; ≥28.5 mg/mL in DMSO |
| Chemical Name | N-[(6-methyl-2-oxo-4-propyl-1H-pyridin-3-yl)methyl]-1-propan-2-yl-6-[6-(4-propan-2-ylpiperazin-1-yl)pyridin-3-yl]indazole-4-carboxamide |
| SDF | Download SDF |
| Canonical SMILES | CCCC1=C(C(=O)NC(=C1)C)CNC(=O)C2=C3C=NN(C3=CC(=C2)C4=CN=C(C=C4)N5CCN(CC5)C(C)C)C(C)C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Scintillation Proximity Assay |
Methyltransferase activity assays are performed by monitoring the incorporation of tritiumlabeled methyl group from S-adenosylmethionine (3H-SAM) to biotinylated peptide substrates using Scintillation Proximity Assay (SPA) for PRC2-EZH2 trimeric complex (EZH2:EED:SUZ12), PRC2-EZH1 pentameric complex (EZH1:EED:SUZ12:RBBP4:AEBP2), SETD7, G9a, GLP, SETDB1, SETD8, SUV420H1, SUV420H2, SUV39H2, MLL1 tetrameric complex (MLL:WDR5:RbBP5:ASH2L), PRMT1, PRMT3, PRMT5-MEP50 complex and SMYD2. The reaction buffer for SMYD2 and SMYD3 is 50 mM Tris pH 9.0, 5 mM DTT, 0.01% TritonX-100; for G9a, GLP and SUV39H2 is 25 mM potassium phosphate pH 8.0, 1 mM EDTA, 2 mM MgCl2 and 0.01% Triton X-100; and for other HMTs 20 mM Tris pH 8.0, 5 mM DTT, 0.01% TritonX-100. To stop the enzymatic reactions, 10 μL of 7.5 M guanidine hydrochloride is added, followed by 180 μL of buffer, mixed and transferred to a 384-well FlashPlate. After mixing, the reaction mixtures are incubated and the CPM counts are measured using Topcount plate reader. The CPM counts in the absence of compound for each data set are defined as 100% activity. In the absence of the enzyme, the CPM counts in each data set are defined as background (0%). IC50 values are determined using compound concentrations ranging from 100 nM to 100 μM. The IC50 values are determined using SigmaPlot software. EZH2-Y641F assays are performed using 30 nM of enzyme in 20 mM Tris pH 8, 5 mM DTT, 0.01% Triton X-100, 5 μM SAM and 1 μM of H3 (1-24) peptide (same as for the wild-type PRC2-EZH2 complex). For DNMT1, the assay is performed using hemimethylated dsDNA as a substrate. The dsDNA substrate is prepared by annealing two complementary strands (biotintlated forward strand: BGAGCCCGTAAGCCCGTTCAGGTCG and reverse strand: CGACCTGAACGGGCTTACGGGCTC), synthesized by Eurofins MWG Operon. Reaction buffer is 20 mM Tris-HCl, pH 8.0, 5mM DTT, 0.01% Triton X-100. Methyltransferase activity assays for DOT1L is performed using Filter-plates. Reaction mixtures in 20 mM Tris-HCl, pH 8.0, 5 mM DTT, 2 mM MgCl2 and 0.01% Triton X-100 are incubated at room temperature for 1h, 100 μL 10% TCA is added, mixed and transferred to filter-plate. Plates are centrifuged at 2000 rpm for 2 min followed by 2 additional 10% TCA wash and one ethanol wash (180 μL) followed by centrifugation. Plates are dried and 100 μL MicroO is added and centrifuged. 70 μL MicroO is added and CPM are measured using Topcount plate reader. |
| Cell experiment [1]: | |
|
Cell lines |
MCF10A cells, Diffused large B cell lymphoma cell lines |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
100 nM-5 μM for 2-8 days |
|
Applications |
UNC1999 concentration-dependently reduced H3K27me3 levels in MCF10A cells and selectively killed diffused large B cell lymphoma cell lines harboring the EZH2Y641N mutant. |
| Animal experiment [1]: | |
|
Animal models |
Male Swiss albino mice model |
|
Dosage form |
15, 50, or 150 mg/kg, intraperitoneal injection or oral administration for 24 h |
|
Applications |
UNC1999 is orally bioavailable and is an useful tool for the biomedical research community to assess long-term therapeutic benefit(s) and potential toxicity in mice. Moreover, UNC1999 (150 mg/kg, intraperitoneal injection) was well tolerated by all test mice. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: 1Konze, K. D., Ma, A., Li, F., Barsyte-Lovejoy, D., Parton, T., Macnevin, C. J., Liu, F., Gao, C., Huang, X. P., Kuznetsova, E., Rougie, M., Jiang, A., Pattenden, S. G., Norris, J. L., James, L. I., Roth, B. L., Brown, P. J., Frye, S. V., Arrowsmith, C. H., Hahn, K. M., Wang, G. G., Vedadi, M. and Jin, J. (2013) An orally bioavailable chemical probe of the Lysine Methyltransferases EZH2 and EZH1. ACS Chem Biol. 8, 1324-1334 |
|
| Description | UNC1999 is a potent, orally bioavailable and selective inhibitor of EZH2 and EZH1 with IC50 values of 2 nM and 45 nM, respectively. | |||||
| Targets | EZH2 | EZH1 | ||||
| IC50 | 2 nM | 45 nM | ||||
Quality Control & MSDS
- View current batch:
Chemical structure
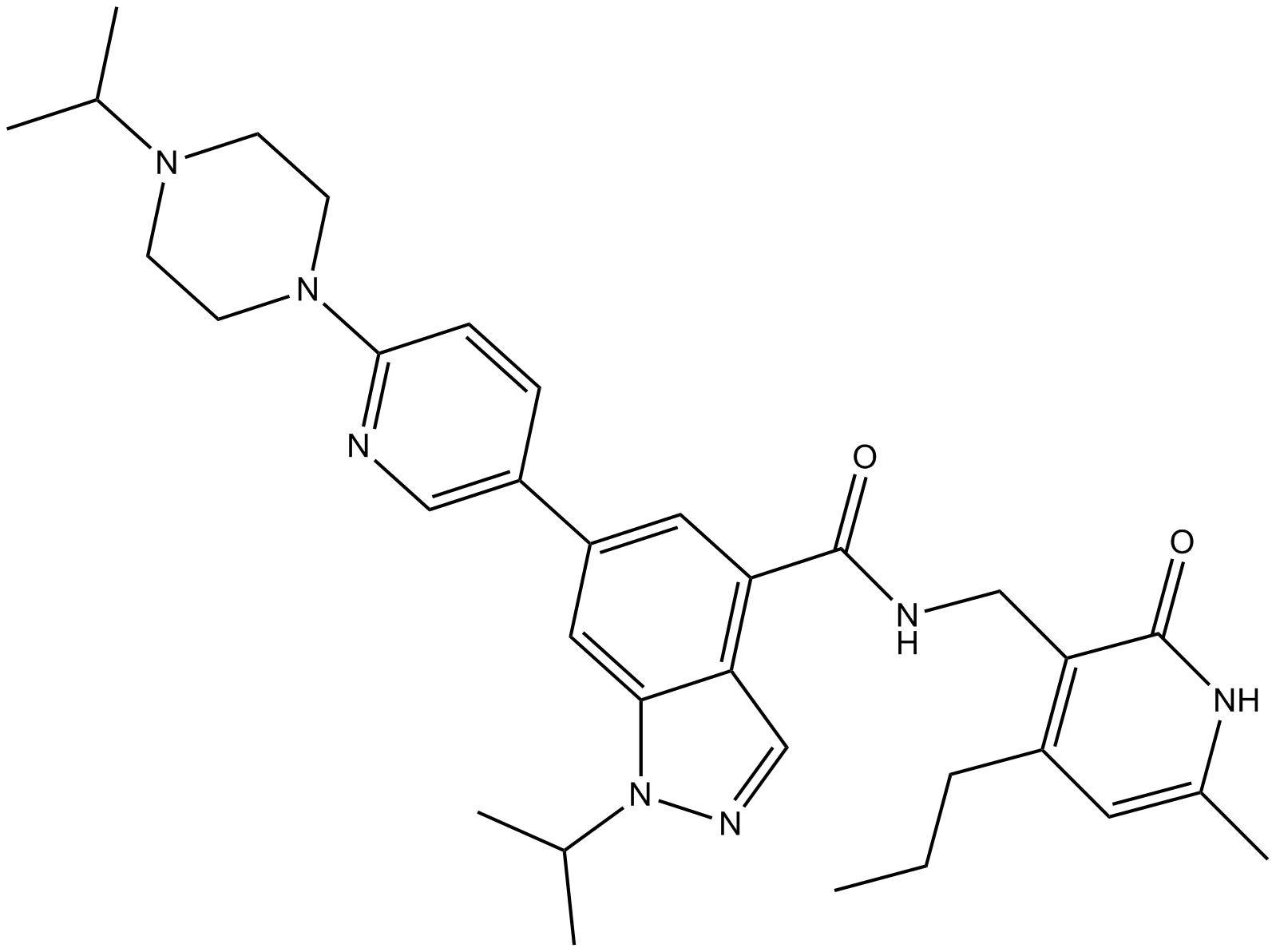
Related Biological Data
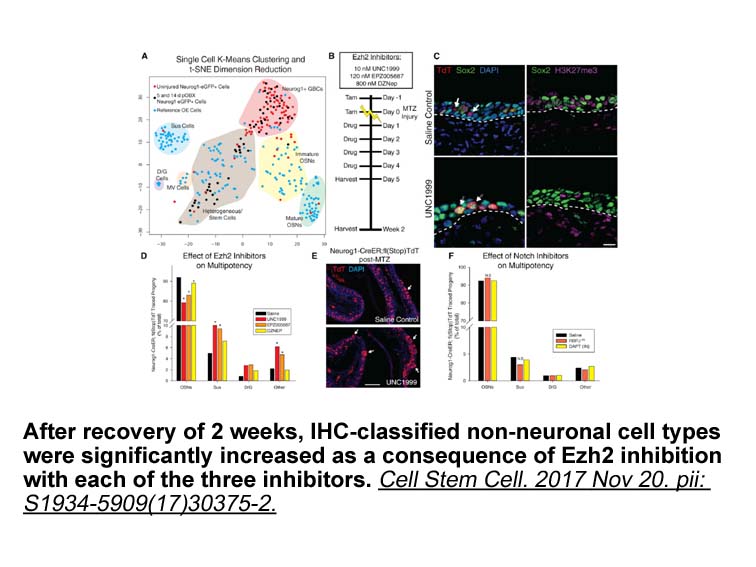
Related Biological Data
Related Biological Data