Ro 31-8220
Ro 31-8220 is a selective inhibitor of protein kinase C (PKC) with IC50 values of 5, 24, 14, 27, and 24 nM for PKC α, PKC βI, PKC βII, PKC γ and PKC ε, respectively [1].
PKC is a monomeric Ca2+- and phospholipid-dependent Ser/Thr protein kinases and plays an important role in growth factor-activated signaling and malignant transformation.
Ro 31-8220 is a selective inhibitor of PKC. Also, Ro 31-8220 increased growth factor-stimulated expression of c-Jun but inhibited MKP-1 and c-Fos expression. Ro-31-8220 strongly stimulated the stress-activated protein kinase JNK1 in a PKC-independent way [2]. In peripheral blood mononuclear cells, Ro 31-8220 inhibited the production of mitogen-induced interleukin (IL) -2 and IL-2-dependent T lymphoblast proliferation with IC50 values of 80 and 350 nM, respectively. Also, Ro 31-8220 (400 nM) inhibited IL-2Rα (CD25) expression by 27%, which suggested that Ro 31-8220 inhibited early and late T cell activation [3].
In rat adipocytes and L6 myotubes, RO 31-8220 activated c-Jun N-terminal kinase (JNK) and glycogen synthase (GS) and stimulated glucose incorporation into glycogen. While, RO 31-8220 inhibited the activity of extracellular response kinases 1 and 2 (ERK1/2) in a PKC-independent way [4].
References:
[1]. Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J, 1993, 294 ( Pt 2): 335-337.
[2]. Beltman J, McCormick F, Cook SJ. The selective protein kinase C inhibitor, Ro-31-8220, inhibits mitogen-activated protein kinase phosphatase-1 (MKP-1) expression, induces c-Jun expression, and activates Jun N-terminal kinase. J Biol Chem, 1996, 271(43): 27018-27024.
[3]. Geiselhart L, Conti DJ, Freed BM. RO 31-8220, a novel protein kinase C inhibitor, inhibits early and late T cell activation events. Transplantation, 1996, 61(11): 1637-1642.
[4]. Standaert ML, Bandyopadhyay G, Antwi EK, et al. RO 31-8220 activates c-Jun N-terminal kinase and glycogen synthase in rat adipocytes and L6 myotubes. Comparison to actions of insulin.
- 1. Manoj Kumar Baniya, Eun-Hee Kim, et al. "Terfenadine, a histamine H1 receptor antagonist, induces apoptosis by suppressing STAT3 signaling in human colorectal cancer HCT116 cells." Front Pharmacol. 2024 Jun 13:15:1418266 PMID: 38939837
- 2. Hai Hu, Mingxing Tian, et al. "Brucellainduces heme oxygenase‐1 expression to promote its infection." Transbound Emerg Dis. 2022 Sep;69(5):2697-2711 PMID: 34918880
| Storage | Store at -20°C |
| M.Wt | 457.55 |
| Cas No. | 125314-64-9 |
| Formula | C25H23N5O2S |
| Solubility | Soluble in DMSO |
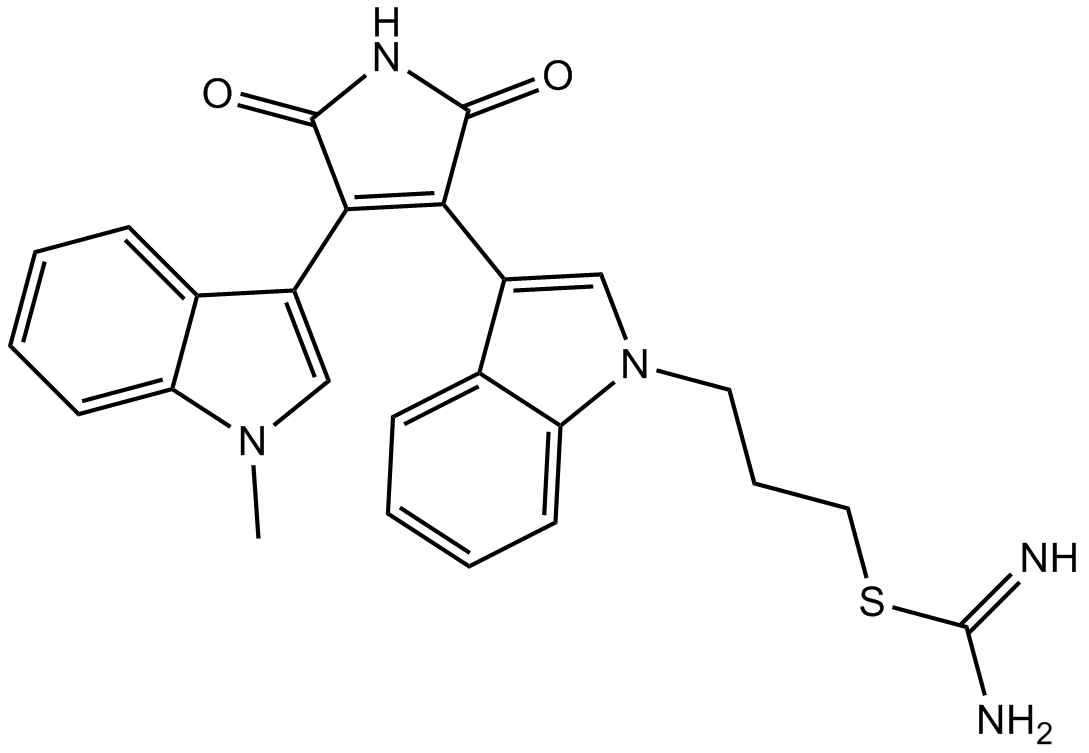
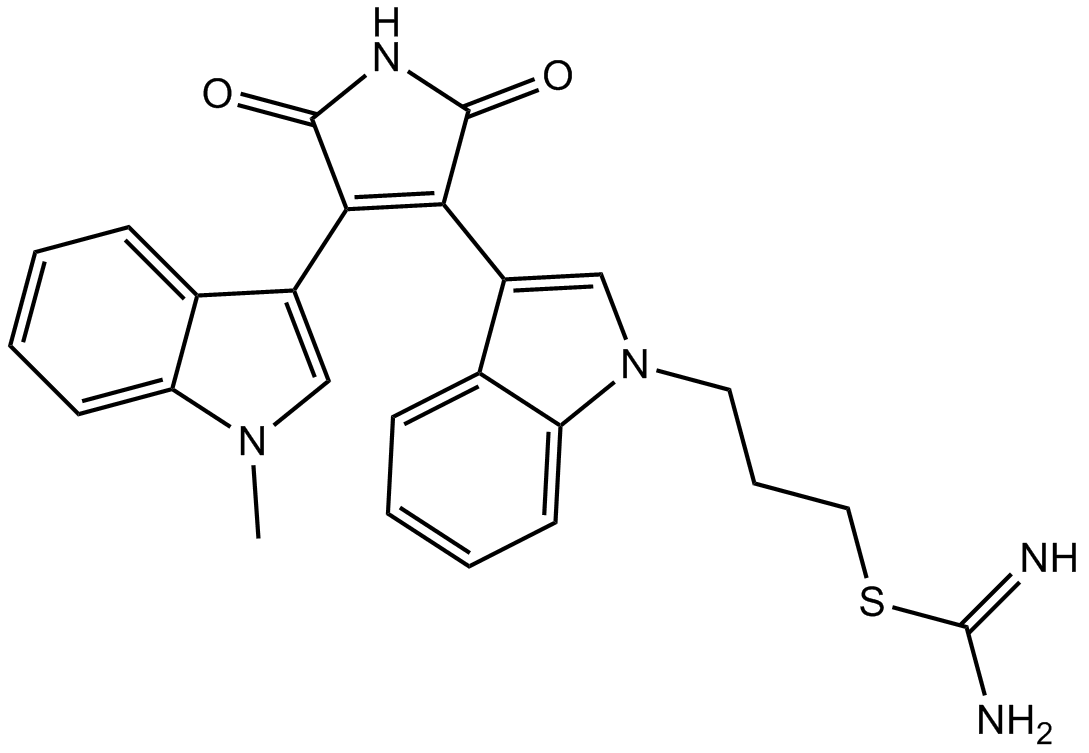
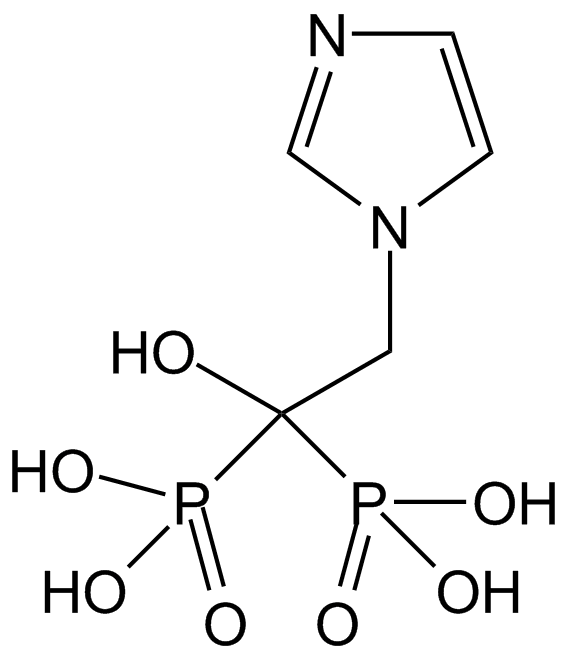
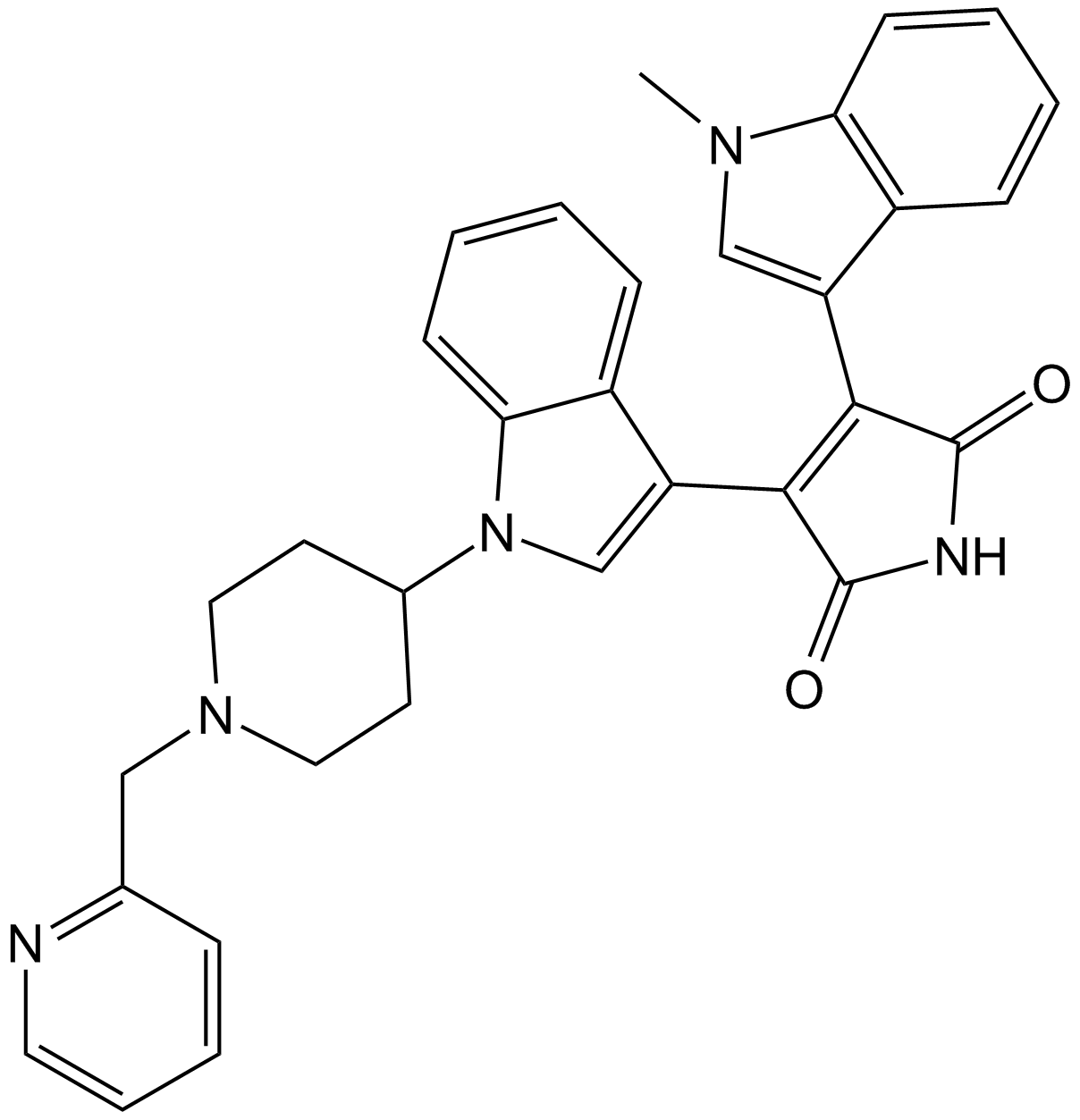
| Chemical Name | 3-[3-[4-(1-methylindol-3-yl)-2,5-dioxopyrrol-3-yl]indol-1-yl]propyl carbamimidothioate |
| SDF | Download SDF |
| Canonical SMILES | C[n]1c(cccc2)c2c(C(C(N2)=O)=C(c3c[n](CCCSC(N)=N)c4c3cccc4)C2=O)c1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Description | Ro 31-8220 is a inhibitor of pan-PKC with IC50 of 5 nM, 24 nM, 14 nM, 27 nM, and 24 nM for PKC-α, PKC-βI, PKC-βII, PKC-γ, and PKC-ε, respectively, | |||||
| Targets | PKC-α | PKC-βII | PKC-βI | PKC-ε | PKC-γ | |
| IC50 | 5 nM | 14 nM | 24 nM | 24 nM | 27 nM | |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure