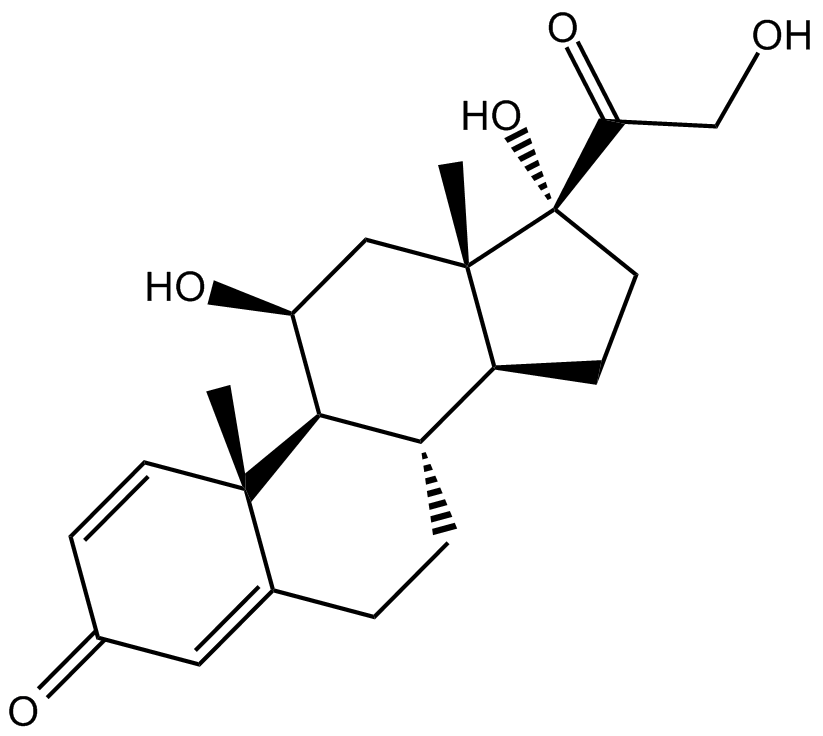
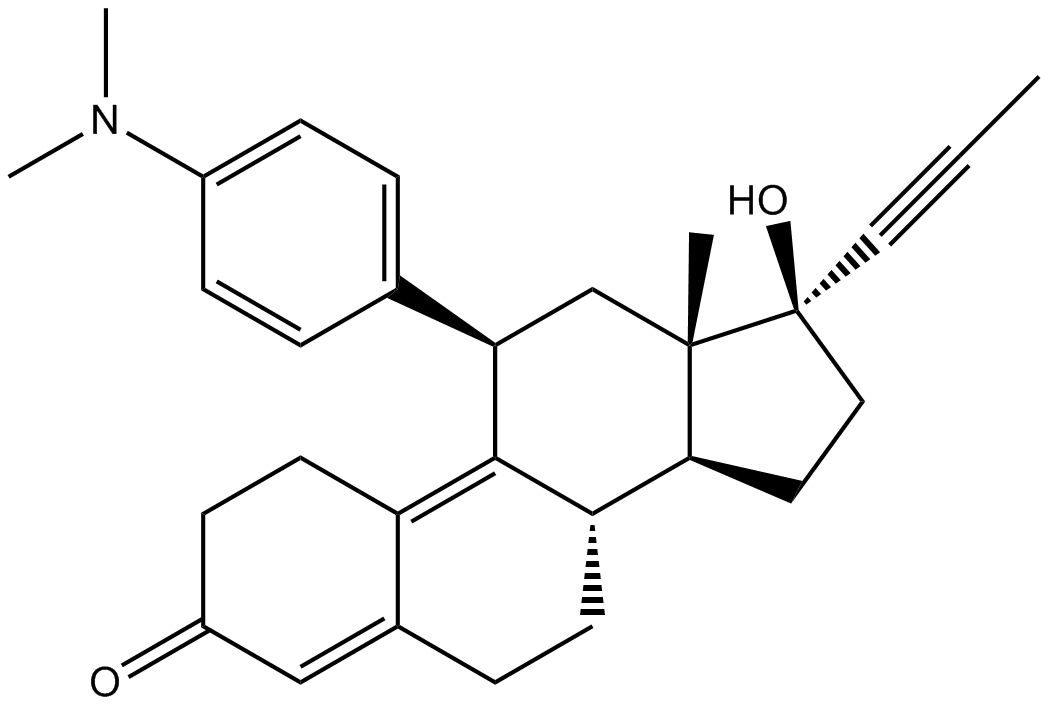
Mifepristone
Mifepristone (RU486) is a potent antagonist of progesterone receptor, used as a contraceptive agent [1].
As a contraceptive agent, Mifepristone has been reported to reduce the size of uterine fibroids, and inhibit the growth of meningioma cells in vitro, in experimental animal models and in patients with inoperable meningiomas. As a non-contraceptive action, Mifepristone was been revealed to inhibit the proliferation of normal and malignant endometrial cells, breast cancer cells, prostate cancer cells and gastric adenocarcinoma cell lines. In addition, Mifepristone has been demonstrated to have progesterone-like effects and suppression ovarian cancer cell growth in a dose-dependent fashion with the IC50 values of 6.25μmol/l and 6.91μmol/l for SK-OV-3 and OV2008 cell lines, respectively [1].
References:
[1] Goyeneche AA1, Carón RW, Telleria CM. Mifepristone inhibits ovarian cancer cell growth in vitro and in vivo. Clin Cancer Res. 2007 Jun 1; 13(11):3370-9.
- 1. Qin Dai, Jinfei Wei, et al. "Mifepristone achieves tumor suppression and ferroptosis through PR/p53/HO1/GPX4 axis in meningioma cells." J Neurooncol. 2025 Jan 3 PMID: 39751705
- 2. Ping Tao, et al. "Nicotine regulates abnormal macrophage polarization and trophoblast invasion associated with preterm labor via the α7nAChR/SIRT1 axis." Placenta. 2024 Mar 6:147:42-51. PMID: 38308901
- 3. Lu Gan, Qiyong Li, et al. "Glucocorticoids rapidly promote YAP phosphorylation via the cAMP-PKA pathway to repress mouse cardiomyocyte proliferative potential." Mol Cell Endocrinol. 2022 Mar 10;548:111615. PMID: 35278645
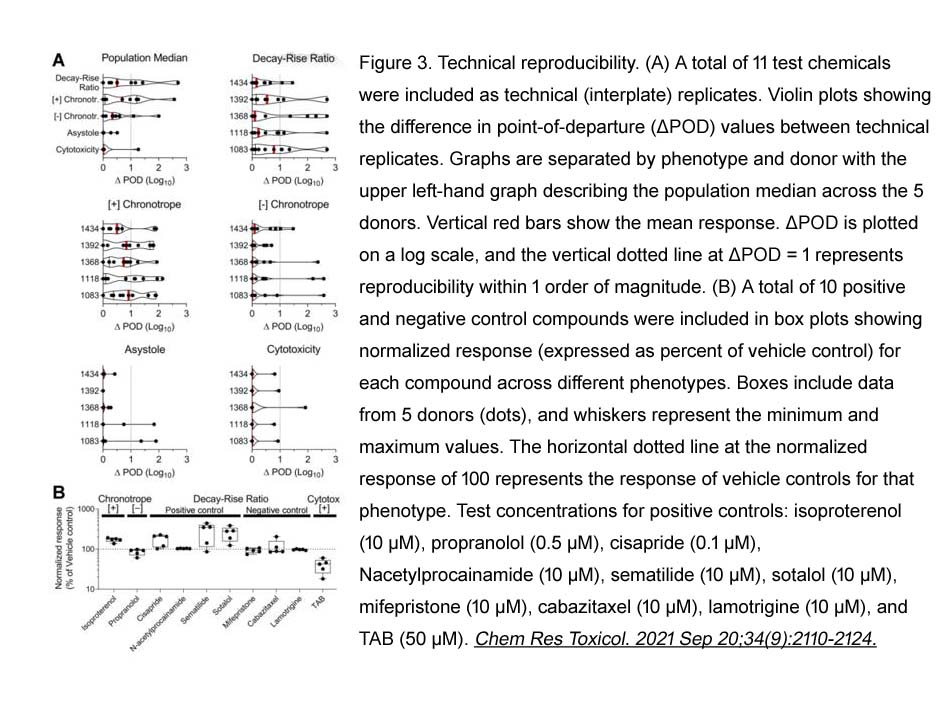
- 4. Sarah D. Burnett, Alexander D. Blanchette, et al. "Cardiotoxicity Hazard and Risk Characterization of ToxCast Chemicals Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes from Multiple Donors." Chem Res Toxicol. 2021 Sep 20;34(9):2110-2124. PMID:34448577
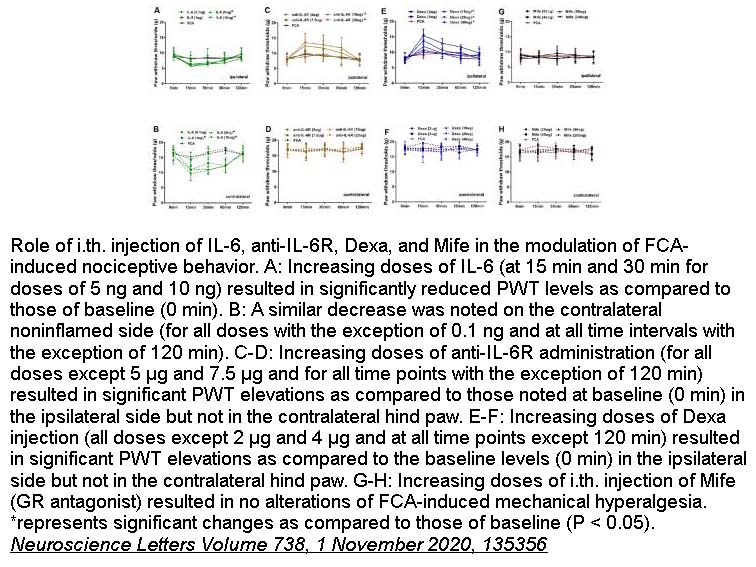
- 5. Xiongjuan Li, Weihong Wang, et al. "Antinociceptive effects of IL-6R vs. glucocorticoid receptors during rat hind paw inflammatory pain." Neuroscience Letters Volume 738, 1 November 2020, 135356. PMID:32898615
- 6. Horchar M, Wohleb ES. "Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress." Brain Behav Immun. 2019 Jun 27. pii: S0889-1591(19)30167-9. PMID:31255679
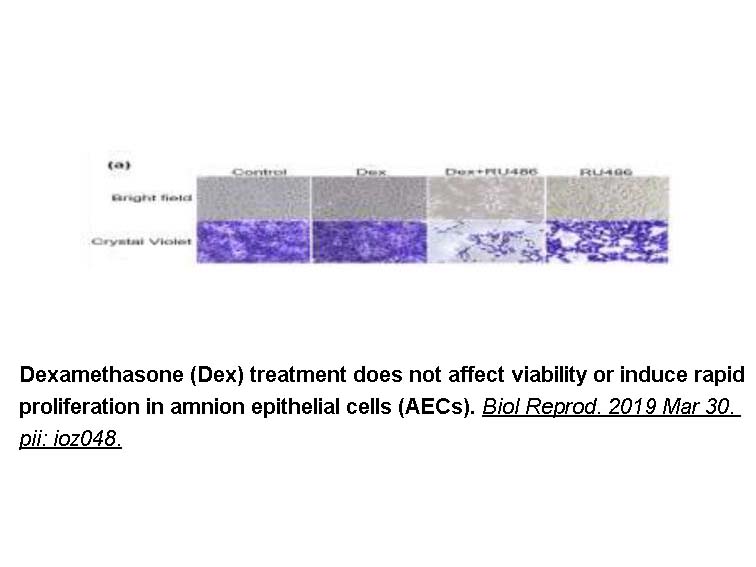
- 7. Martin LF, Richardson LS, et al. "Dexamethasone induces primary amnion epithelial cell senescence through telomere-P21 associated pathway†." Biol Reprod. 2019 Jun 1;100(6):1605-1616. PMID:30927408
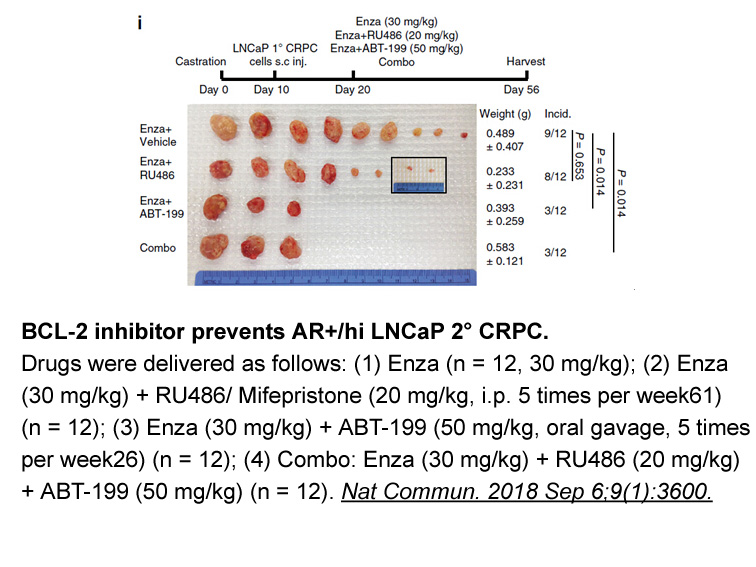
- 8. Li Q, Deng Q, et al. "Linking prostate cancer cell AR heterogeneity to distinct castration and enzalutamide responses." Nat Commun. 2018 Sep 6;9(1):3600. PMID:30190514
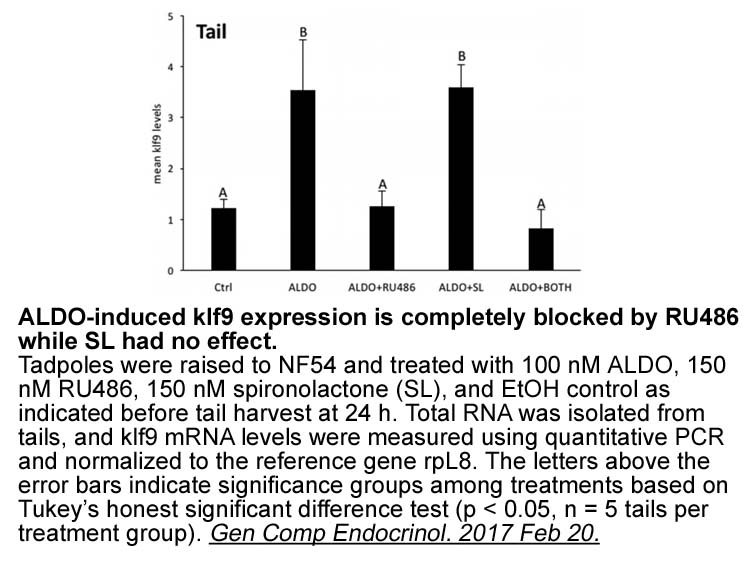
- 9. Shewade LH, Schneider KA, et al. "In-vivo regulation of Krüppel-like factor 9 by corticosteroids and their receptors across tissues in tadpoles of Xenopus tropicalis. "Gen Comp Endocrinol. 2017 Feb 20. pii: S0016-6480(16)30412-9. PMID:28232027
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 429.59 |
| Cas No. | 84371-65-3 |
| Formula | C29H35NO2 |
| Solubility | ≥21.48 mg/mL in DMSO; insoluble in H2O; ≥21.45 mg/mL in EtOH with gentle warming |
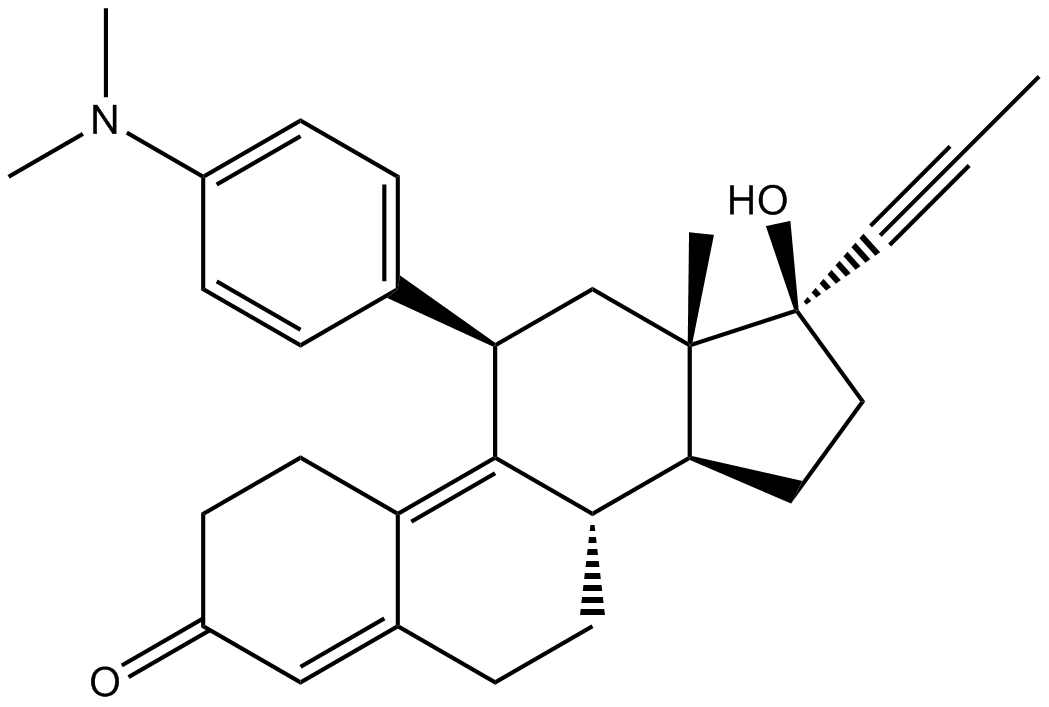
| Chemical Name | (8S,11R,13S,14S,17S)-11-[4-(dimethylamino)phenyl]-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one |
| SDF | Download SDF |
| Canonical SMILES | C[C@](C[C@@H]1c(cc2)ccc2N(C)C)([C@@H](CC2)[C@H](CC3)C1=C(CC1)C3=CC1=O)[C@@]2(C#CC)O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Glucocorticoid receptor (GR) antagonist activity, Progesterone receptor (PR) antagonist activity |
T47D alkaline phosphatase assay: T47D human breast cancer cells are plated in 96 well tissue culture plates at 104 cells per well in assay medium [RPMI medium without phenol red containing 5% (v/v) charcoal treated FBS and 1% (v/v) penicillin streptomycin]. Two days later, the medium is decanted and Mifepristone or control is added at a final concentration of 0.1% (v/v) dimethylsulfoxide in fresh assay medium. Twenty four hours later, an alkaline phosphatase assay is performed using a SEAP kit. The medium is decanted and the cells are fixed for 30 minutes at room temperature with 5% (v/v) formalin. The cells are washed once at room temperature with Hanks' buffered saline solution. Equal volumes (0.05 mL) of dilution buffer, assay buffer, and 1:20 substrate/enhancer mixture are then added. After 1 hour incubation in the dark at room temperature, the lysate is transferred to a white 96 well plate and luminescence is read using a LuminoSkan Ascen. A549 reporter assay: A549 human lung carcinoma cells are washed with Opti-MEM I. The medium is removed and lipid DNA complex solution (1.5 μg/mL of GRE luciferase reporter DNA in 8.5 mL Opti-MEM I plus 6 μL/mL DMRIE-C reagent in 8.5 mL Opti-MEM I, combined, mixed and incubated at room temperature for 40 minutes) is overlayed onto the cells in a T160 flask. The cells are incubated for 16 hours at 37℃ in a CO2 incubator. The DNA containing medium is removed and 30 mL of growth medium containing 5% (v/v) charcoal treated fetal bovine serum is added. After 5-6 hours, the cells are seeded in 96 well plates and incubated overnight at 37℃. Mifepristone is then added to each well followed by dexamethasone as a corticoid challenge. The cells are incubated for 24 hours. Luciferase assay buffer is added to each well and the cells are incubated for 30 minutes at room temperature. Luciferase activity is measured in a DYNEX Microlite plate on a TopCount. |
| Cell experiment: | |
|
Cell lines |
Sperm; ovarian cancer cell lines (SK-OV-3, Caov-3, IGROV-1, and OV2008 cells) |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0.04, 0.4, 4 and 40 μM for 1 h; or 20 μM for 1-3 days |
|
Applications |
Mifepristone modulated human sperm functions and dose-dependently inhibited progesterone-induced acrosome reaction, hyperactivation and [Ca2+]i in human spermatozoa [2]. Moreover, mifepristone suppressed cell growth and decreased the expression of the S phase cyclin (cyclin A) and the M phase cyclin (cyclin B1) in various ovarian cancer cell lines [3]. |
| Animal experiment: | |
|
Animal models |
SK-OV-3 tumor xenografts model |
|
Dosage form |
0.5, 1.0 mg/d, s.c., for 40 days |
|
Applications |
Mifepristone (0.5, 1.0 mg/d) dose-dependently inhibited SK-OV-3 tumors growth in nude mice [3]. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: 1. Jiang, W., Allan, G., Fiordeliso, J. J., Linton, O., Tannenbaum, P., Xu, J., Zhu, P., Gunnet, J., Demarest, K., Lundeen, S. and Sui, Z. (2006) New progesterone receptor antagonists: phosphorus-containing 11beta-aryl-substituted steroids. Bioorg Med Chem. 14, 6726-6732 2. Ko, J. K., Huang, V. W., Li, R. H., Yeung, W. S., Ho, P. C. and Chiu, P. C. (2014) An in vitro study of the effect of mifepristone and ulipristal acetate on human sperm functions. Andrology. 2, 868-874 3. Goyeneche, A. A., Caron, R. W. and Telleria, C. M. (2007) Mifepristone inhibits ovarian cancer cell growth in vitro and in vivo. Clin Cancer Res. 13, 3370-3379 |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data

Related Biological Data
Related Biological Data