G007-LK
G007-LK is a potent and specific inhibitor of tankyrase 1/2 with IC50 values of 46 and 25 nM [1].
The telomeric repeat factor 1 (TRF1)-interacting ankyrin-related ADP-ribose polymerase 1 (tankyrase 1,TNKS1) and tankyrase 2 (TNKS2) belong to the subgroup of poly(ADP-ribosyl)ating polymerases and regulate the assembly and disassembly of large polymerized structures [1].
G007-LK is a potent and specific tankyrase 1/2 inhibitor. G007-LK reduced auto-poly-(ADP ribosy)lation of TNKS1 and TNKS2 with IC50 values of 46 nM and 25 nM, respectively. In Wnt3a-induced HEK 293 cells, G007-LK inhibited ST-Luc with IC50 value of 0.05 μM [1]. In SW480 colorectal cancer cell line transfected with GFP-TNKS1, G007-LK induces highly dynamic and mobile degradasomes containing phosphorylated beta-catenin, beta-TrCP and ubiquitin [2]. In the APC-mutant cell lines, G007-LK reduces cytosolic and nuclear β-catenin protein levels [3].
In mice bearing COLO-320DM cell xenografts, G007-LK (20 mg/kg twice daily or 40 mg/kg daily) concentration-dependently inhibited tumor growth by 61% and 48%, respectively. Also, G007-LK reduced the levels of TNKS1/2 and β-catenin, and stabilized AXIN1/2 [3].
References:
[1]. Voronkov A, Holsworth DD, Waaler J, et al. Structural basis and SAR for G007-LK, a lead stage 1,2,4-triazole based specific tankyrase 1/2 inhibitor. J Med Chem, 2013, 56(7): 3012-3023.
[2]. Thorvaldsen TE, Pedersen NM, Wenzel EM, et al. Structure, Dynamics and Functionality of Tankyrase Inhibitor-induced Degradasomes. Mol Cancer Res, 2015, pii: molcanres.0125.2015.
[3]. Lau T, Chan E, Callow M, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res, 2013, 73(10): 3132-3144.
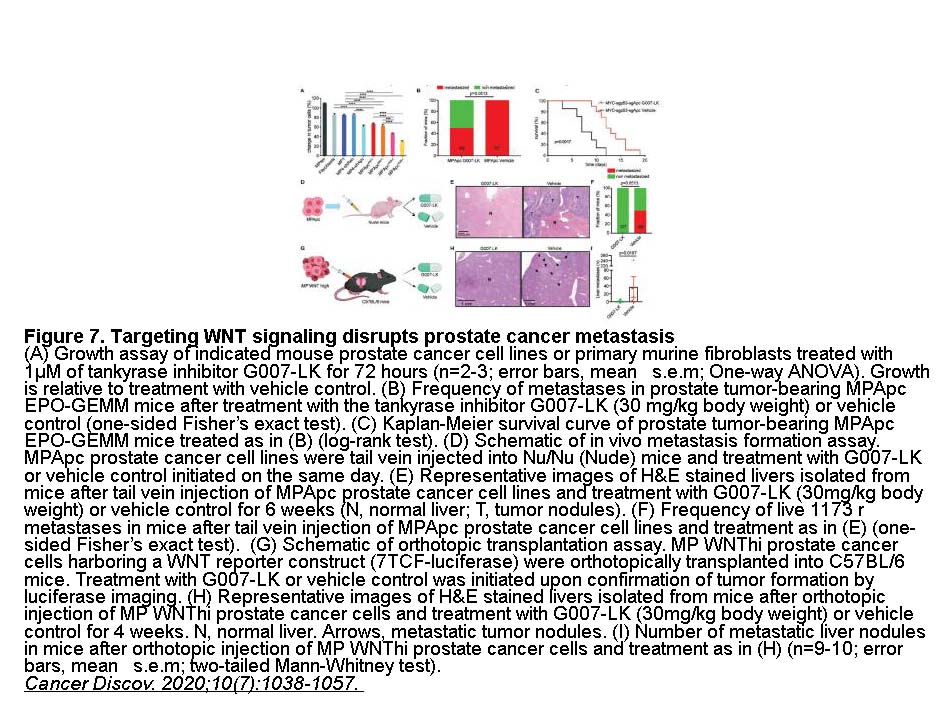
- 1. Leibold J, Ruscetti M, et al. "Somatic Tissue Engineering in Mouse Models Reveals an Actionable Role for WNT Pathway Alterations in Prostate Cancer Metastasis." Cancer Discov. 2020;10(7):1038-1057.nbsp;PMID:32376773
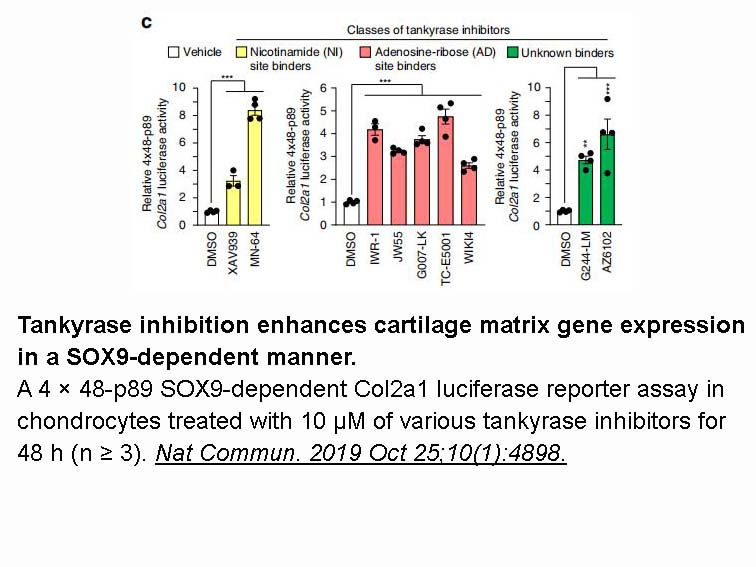
- 2. Kim S, Han S, et al. "Tankyrase inhibition preserves osteoarthritic cartilage by coordinating cartilage matrix anabolism via effects on SOX9 PARylation." Nat Commun. 2019 Oct 25;10(1):4898.nbsp;PMID:31653858
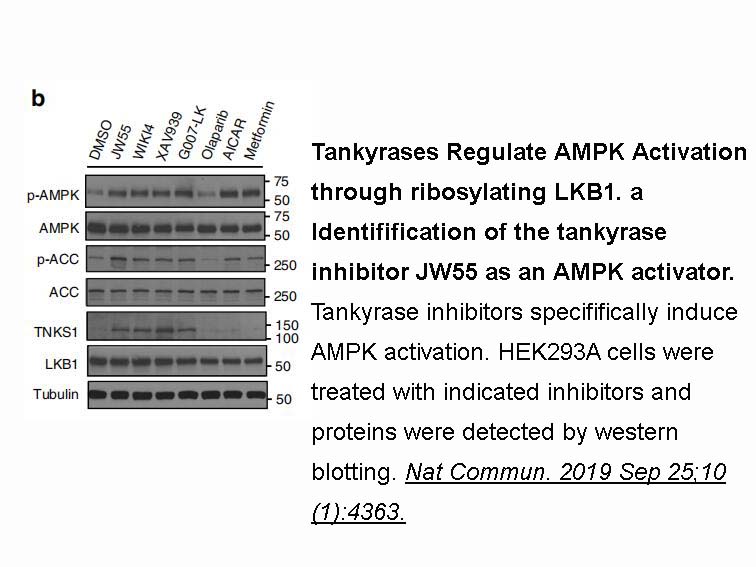
- 3. Li N, Wang Y, et al. "Tankyrase disrupts metabolic homeostasis and promotes tumorigenesis by inhibiting LKB1-AMPK signalling." Nat Commun. 2019 Sep 25;10(1):4363.nbsp;PMID:31554794
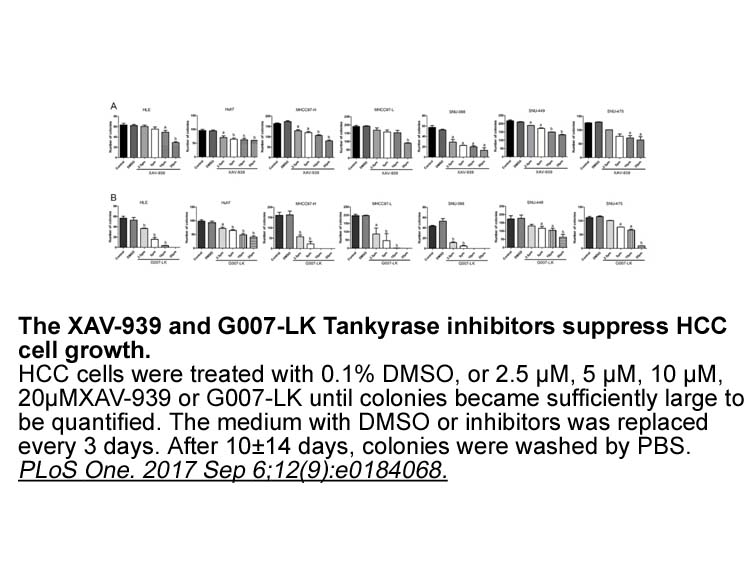
- 4. Jia J, Qiao Y, et al. "Tankyrase inhibitors suppress hepatocellular carcinoma cell growth via modulating the Hippo cascade." PLoS One. 2017 Sep 6;12(9):e0184068. PMID:28877210
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 529.96 |
| Cas No. | 1380672-07-0 |
| Formula | C25H16ClN7O3S |
| Solubility | ≥26.5 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
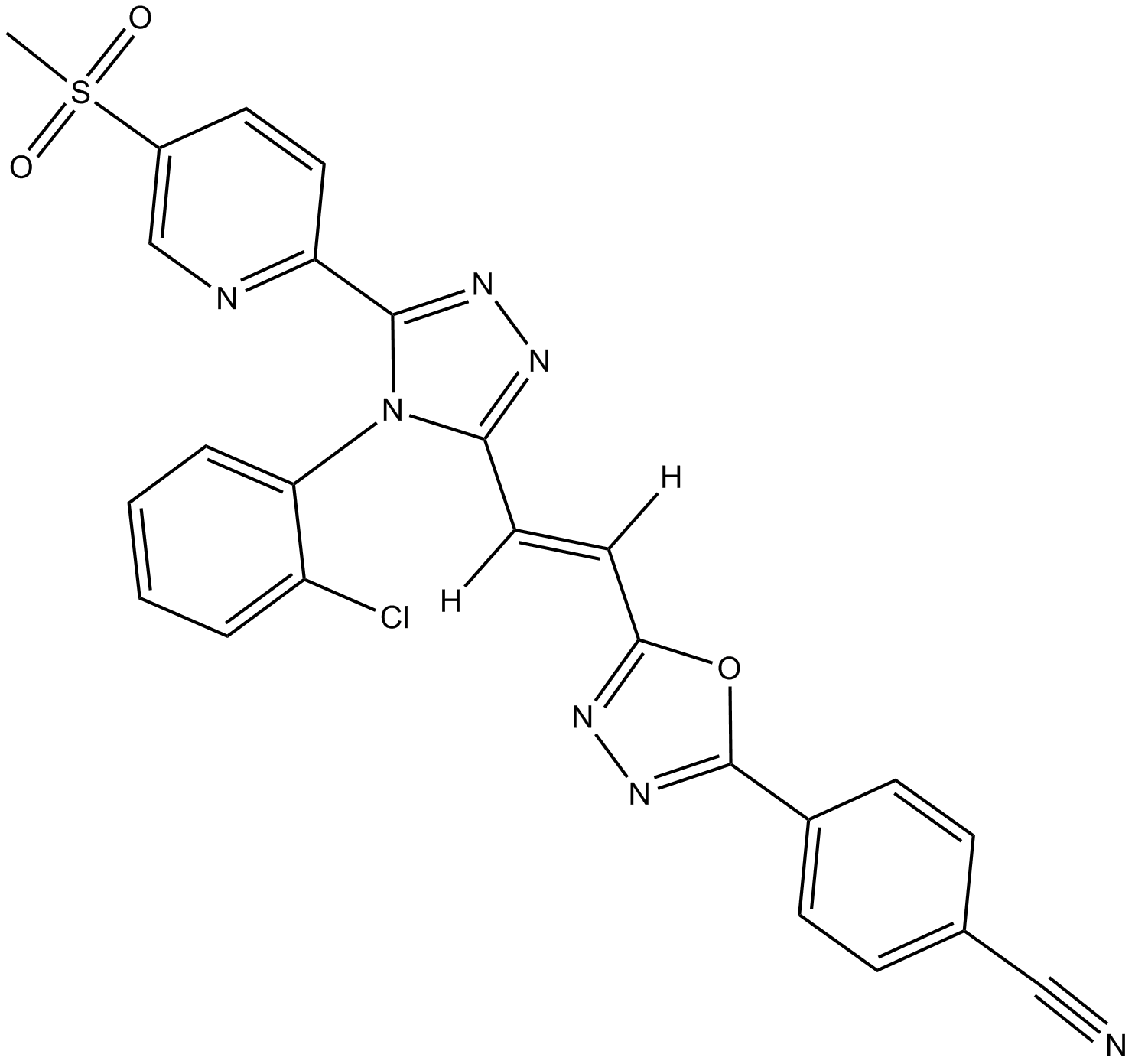
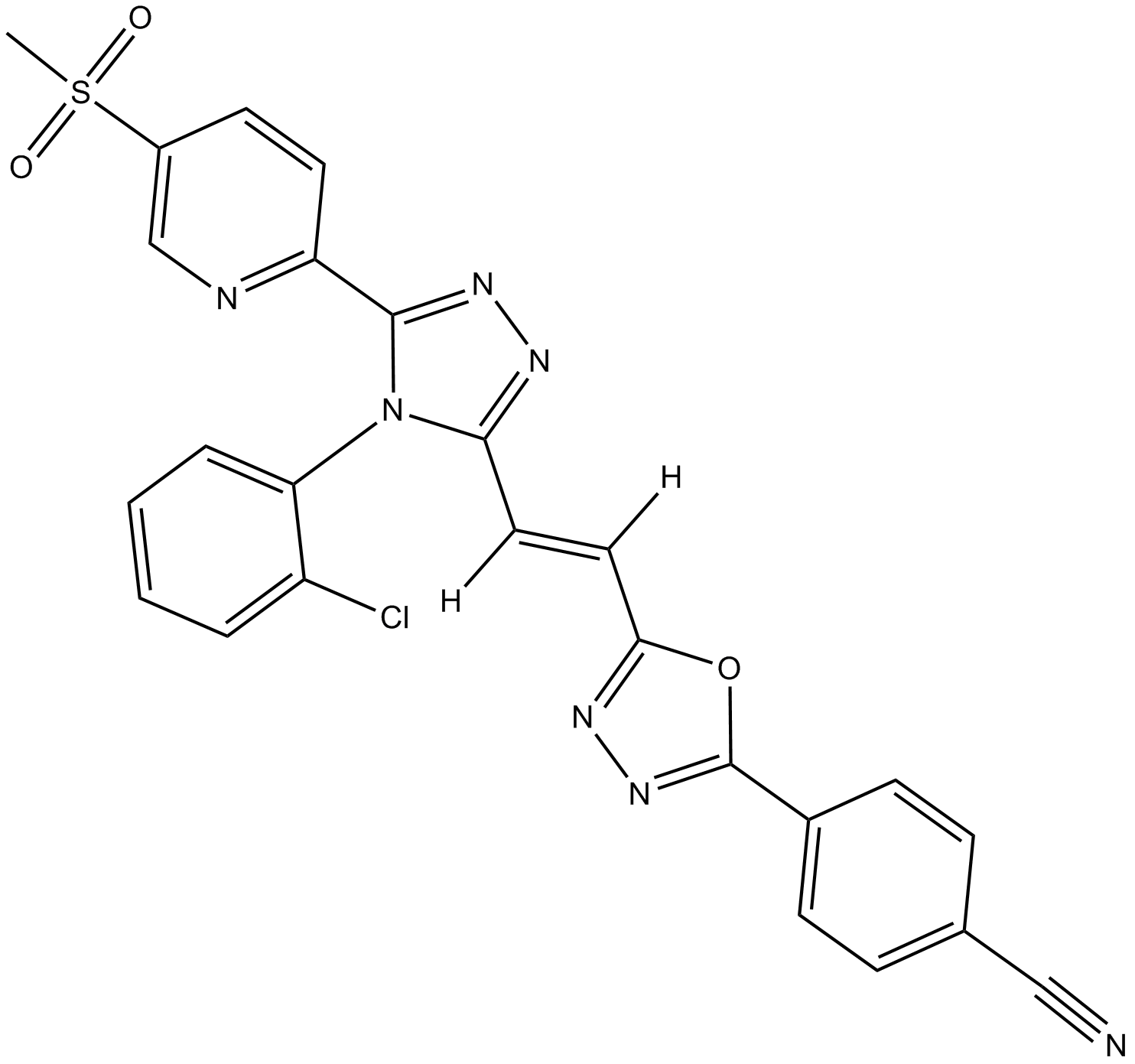
| Chemical Name | (E)-4-(5-(2-(4-(2-chlorophenyl)-5-(5-(methylsulfonyl)pyridin-2-yl)-4H-1,2,4-triazol-3-yl)vinyl)-1,3,4-oxadiazol-2-yl)benzonitrile |
| SDF | Download SDF |
| Canonical SMILES | CS(C1=CN=C(C2=NN=C(N2C3=CC=CC=C3Cl)/C([H])=C([H])/C(O4)=NN=C4C5=CC=C(C#N)C=C5)C=C1)(=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
COLO-320DM cells lines |
|
Preparation method |
The solubility of this compound in DMSO is >26.5mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0.2 μmol/L for 7 to 13 days |
|
Applications |
G007-LK inhibits cell-cycle progression, reduces colony formation, and induces differentiation, suggesting that b-catenin–dependent maintenance of an undifferentiated state may be blocked by tankyrase inhibition. |
| Animal experiment [1]: | |
|
Animal models |
Xenograft COLO-320DM tumors mice |
|
Dosage form |
20, 40, 60 mg/kg, IP, daily for 21 days |
|
Application |
G007-LK showed antitumor efficacy for Xenograft COLO-320DM tumors mice. TNKS1 and TNKS2 protein levels were reduced, AXIN1 and AXIN2 were stabilized, and b-catenin level was decreased. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Lau T, Chan E, Callow M, Waaler J, et. al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013 May 15;73(10):3132-44. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data