Fludarabine Phosphate (Fludara)
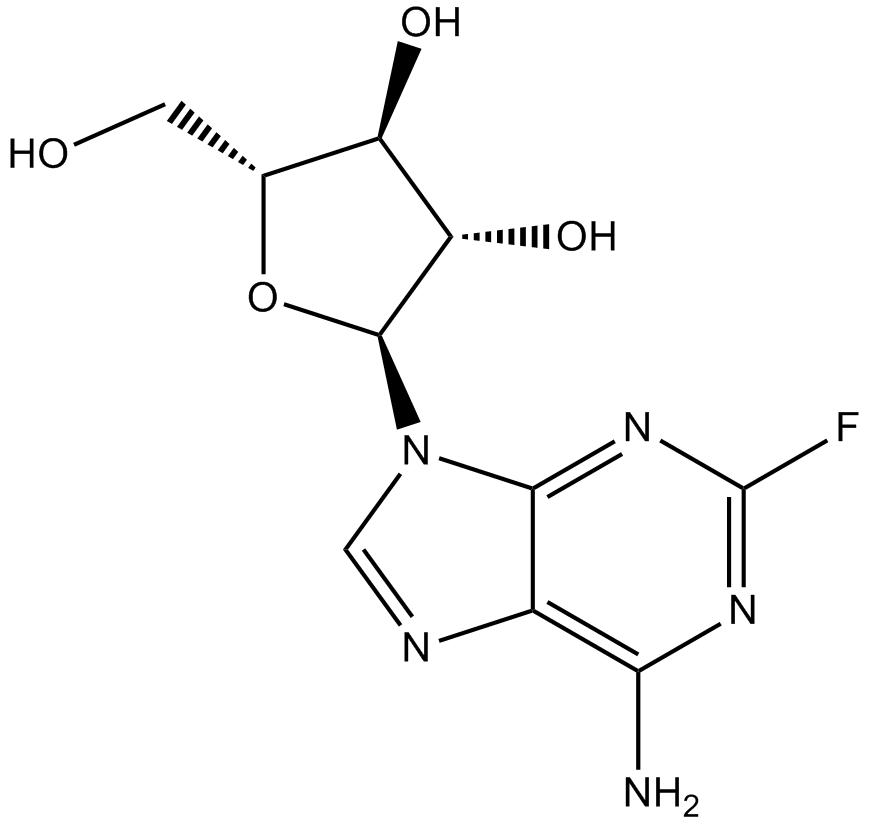
Fludarabine phosphate (CAS 75607-67-9), marketed as Fludara, is a fluorinated nucleotide analog derived from vidarabine (ara-A), exhibiting antineoplastic activities by interfering with DNA synthesis. Following rapid extracellular dephosphorylation to 2-fluoro-ara-A, this metabolite is intracellularly phosphorylated by deoxycytidine kinase, generating the active triphosphate metabolite 2-fluoro-ara-ATP. The active metabolite inhibits critical enzymes including DNA polymerase alpha, ribonucleotide reductase, and DNA primase, thereby disrupting DNA replication and ultimately impeding tumor cell proliferation. This compound is broadly utilized in biomedical research focused on anticancer mechanisms and nucleotide metabolism studies.
- 1. Zhiwei Fan, Yiting Liu, et al. "APOL6 predicts immunotherapy efficacy of bladder cancer by ferroptosis." BMC Cancer. 2024 Aug 26;24(1):1046. PMID: 39187773
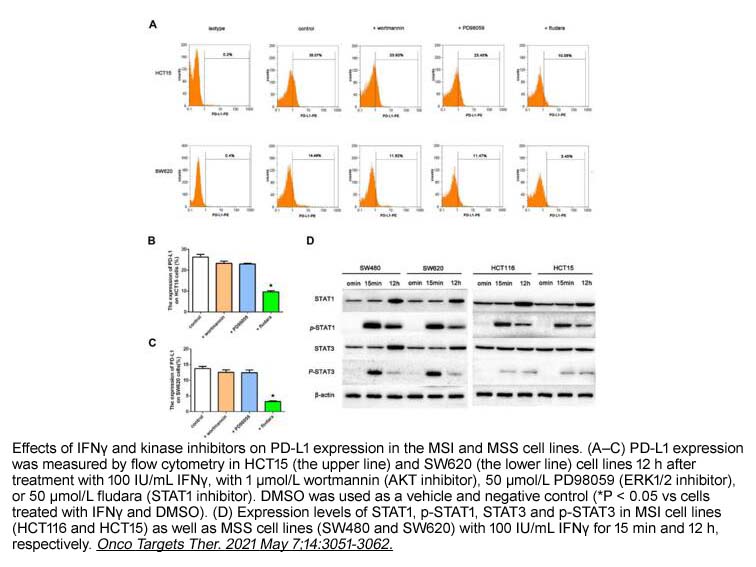
- 2. Wenli Yuan, Deyao Deng, et al. "IFNγ/PD-L1 Signaling Improves the Responsiveness of Anti-PD-1 Therapy in Colorectal Cancer: An in vitro Study." Onco Targets Ther. 2021 May 7;14:3051-3062. PMID:33994797
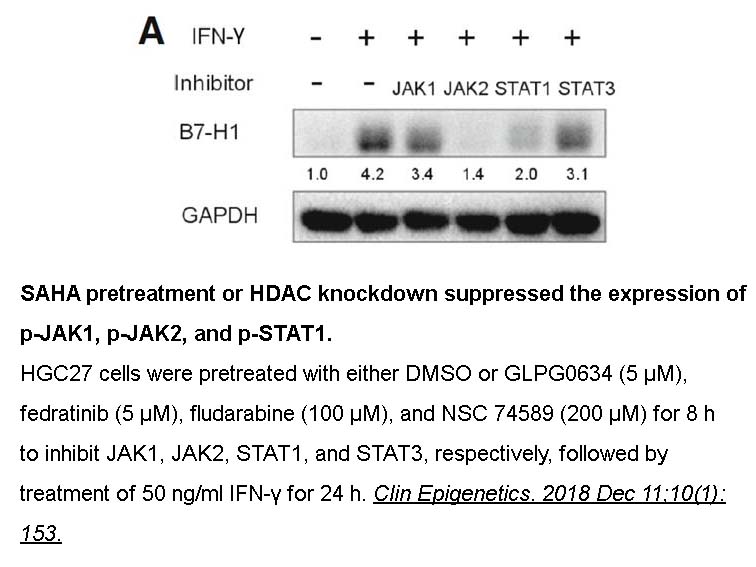
- 3. Diqi Yang, Ai Liu, et al. "BCL2L15 Depletion Inhibits Endometrial Receptivity via the STAT1 Signaling Pathway." Genes (Basel). 2020 Jul 17;11(7):816. PMID:32708974
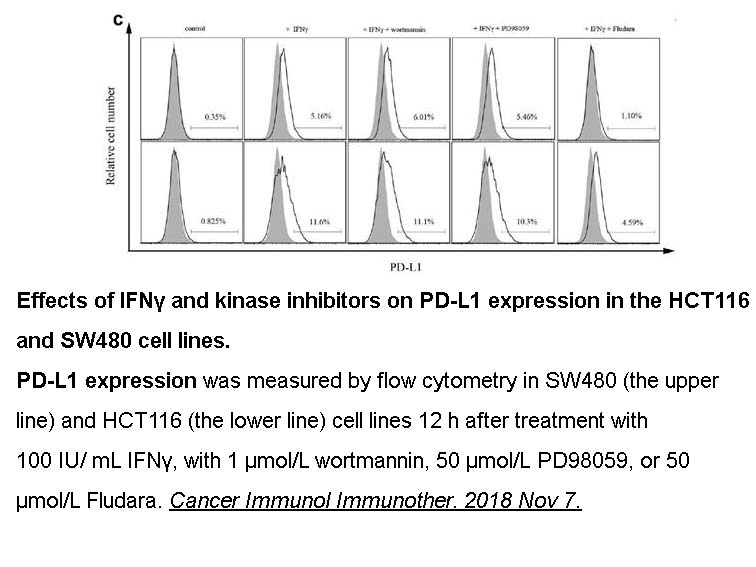
- 4. Deng R, Zhang P, et al. "HDAC is indispensable for IFN-γ-induced B7-H1 expression in gastric cancer." Clin Epigenetics. 2018 Dec 11;10(1):153. PMID:30537988
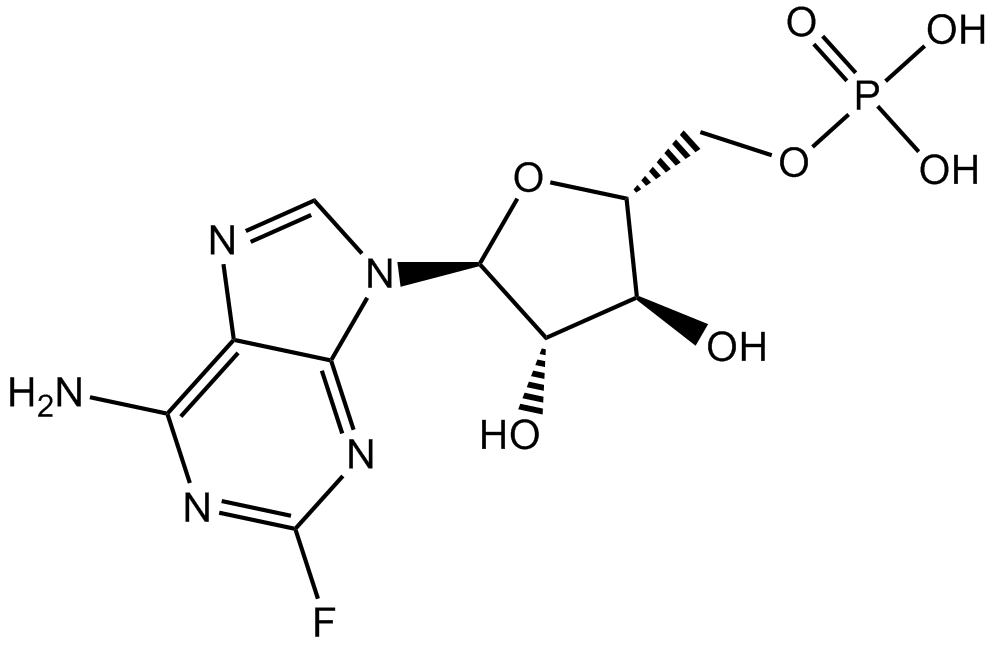
- 5. Yuan W, Deng D,et al."Hyperresponsiveness to interferon gamma exposure as a response mechanism to anti-PD-1 therapy in microsatellite instability colorectal cancer." Cancer Immunol Immunother. 2018 Nov 7. PMID:30406373
| Storage | Store at -20°C |
| M.Wt | 365.21 |
| Cas No. | 75607-67-9 |
| Formula | C10H13FN5O7P |
| Synonyms | Fludura |
| Solubility | insoluble in EtOH; ≥17.6 mg/mL in DMSO; ≥6.7 mg/mL in H2O with gentle warming and ultrasonic |
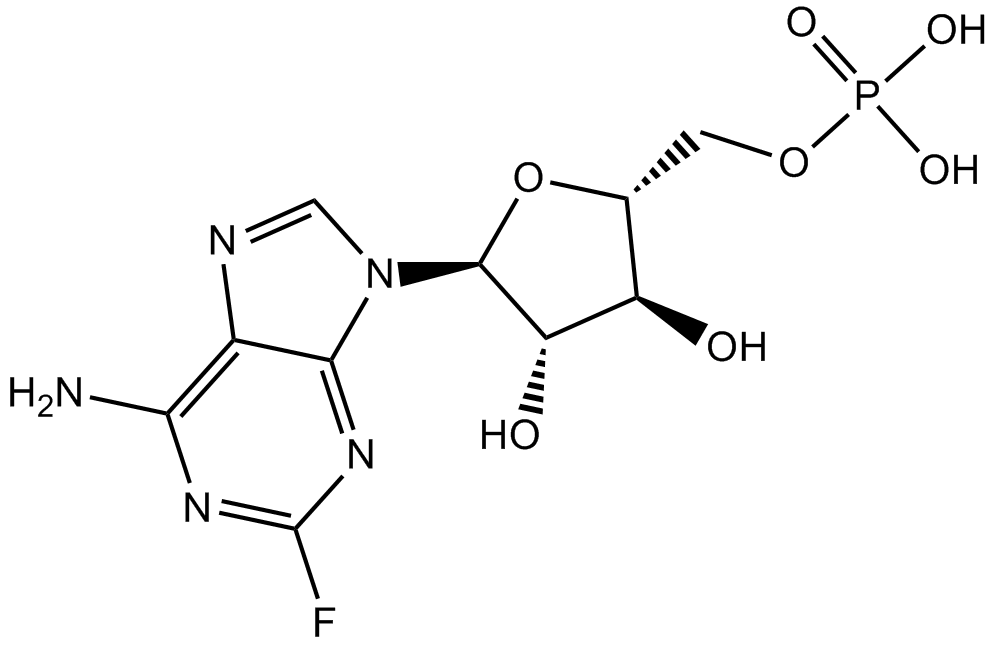
| Chemical Name | [(2R,3S,4S,5R)-5-(6-amino-2-fluoropurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate |
| SDF | Download SDF |
| Canonical SMILES | Nc1c2nc[n]([C@@H]([C@H]3O)O[C@H](COP(O)(O)=O)[C@H]3O)c2nc(F)n1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[1] | |
|
Cell lines |
Human T lymphoblastoid cells, CCRF-CEM |
|
Reaction Conditions |
0.3 ~ 100 μM fludarabine phosphate for 5 h incubation |
|
Applications |
Fludarabine phosphate (0.3 ~ 100 μM) was dose-dependently converted to F-ara-ATP in cells. However, the incorporation of F-ara-ATP into DNA was self-limited. At 0.3 ~ 10 μM fludarabine phosphate, the amount of the F-ara-AMP incorporated into DNA increased in a dose-dependent manner, but could not further increased at fludarabine phosphate concentrations greater than 10 μM. |
| Animal experiment:[2] | |
|
Animal models |
Mice bearing P388 leukemia |
|
Dosage form |
234 mg/kg Injected intraperitoneally |
|
Applications |
Fludarabine phosphate administered as a single dose resulted in fewer cells surviving therapy in mice bearing P388 leukemia, accompanied by a greater percentage of increase in life span (110%) and increased median survival time. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Huang P, Chubb S, Plunkett W. Termination of DNA synthesis by 9-beta-D-arabinofuranosyl-2-fluoroadenine. A mechanism for cytotoxicity. Journal of Biological Chemistry, 1990, 265(27): 16617-16625. 2. Avramis VI, Plunkett W. Metabolism and therapeutic efficacy of 9-beta-D-arabinofuranosyl-2-fluoroadenine against murine leukemia P388. Cancer Research, 1982, 42(7): 2587-2591. |
|
| Description | Fludarabine is an inhibitor of STAT1 activation and a DNA synthesis. | |||||
| Targets | DNA synthesis | |||||
| IC50 | ||||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data