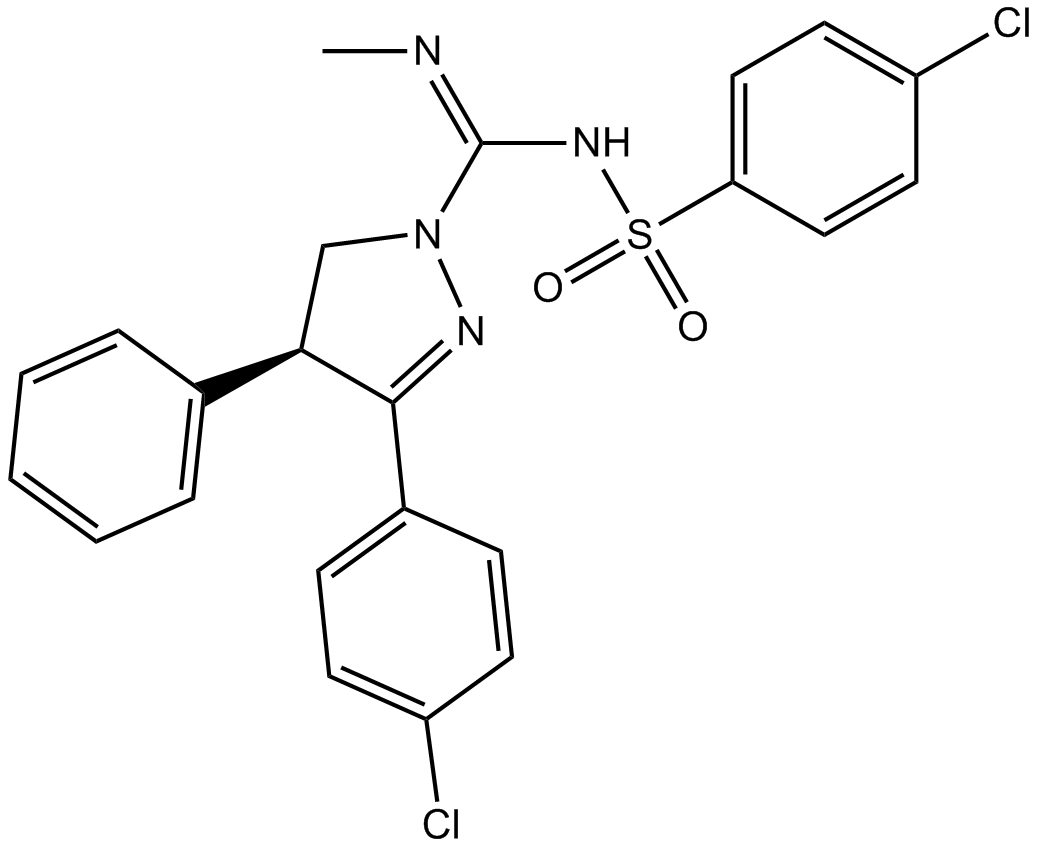
(±)-SLV 319
SLV 319 is described here instead of (±)-SLV 319. SLV 319, also called ibipinabant, is a CB1 antagonist with an IC50 value of 22 nM [1, 2].
There are 2 types of cannabinoid receptors (CB1 and CB2). Their endogenous ligands are primarily anandamide and 2-AG. Cannabinoid receptors and their endogenous ligands together are prominent in the control of food intake and energy metabolism. Stimulation of this endocannabinoid system triggers metabolic processes and leads to lipogenesis, weight gain, insulin resistance, dyslipidemias and impaired glucose homeostasis [2].
C2C12 murine myoblasts were model cells. Exposure to increasing concentrations of ibipinabant at the highest concentration tested (100 μM) for 24 hours significantly decreased cell viability to 73 ± 5%. After 48 hours of exposure to the drug at this concentration only 33 ± 4% of cells remained viable. Cellular reactive oxygen species (ROS) generation is an index of mitochondrial function. A more than 2-fold increase in cellular ROS generation could already be observed after 8 hours exposure to ibipinabant at a concentration of 100 μM compared to the vehicle treated C2C12 myoblasts [3].
CB1 receptor occupancy was related to potencies to increase dopamine (DA) and norepinephrine (NE) releases. In the medial prefrontal cortex (mPFC), SLV 319 caused a significant increase in the extracellular DA level at 10 mg/kg. The time course of the effects of SLV 319 and SR141716A at the 10-mg/kg dose appeared to be similar with peak effects at 30 and 180 min. But SLV 319 showed a more pronounced biphasic effect on DA release. SLV319 administration caused significant increases in extracellular concentrations of 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), metabolites of DA and NE, after the 10-mg/kg dose in rat [4].
References:
[1]. Srivastava BK, Soni R, Joharapurkar A, et al. Bioisosteric replacement of dihydropyrazole of 4S-(-)-3-(4-chlorophenyl)-N-methyl-N’-[(4-chlorophenyl)-
sulfonyl]-4-phenyl-4,5-dihydro-1H-pyrazole-1-caboxamidine (SLV-319) a potent CB1 receptor antagonist by imidazole and oxazole. Bioorganic & medicinal chemistry letters, 2008, 18(3): 963-968.
[2]. Chorvat RJ, Berbaum J, Seriacki K, et al. JD-5006 and JD-5037: peripherally restricted (PR) cannabinoid-1 receptor blockers related to SLV-319 (Ibipinabant) as metabolic disorder therapeutics devoid of CNS liabilities. Bioorganic & medicinal chemistry letters, 2012, 22(19): 6173-6180.
[3]. Schirris TJJ, Ritschel T, Renkema GH, et al. Mitochondrial ADP/ATP exchange inhibition: a novel off-target mechanism underlying ibipinabant-induced myotoxicity. Scientific reports, 2015, 5.
[4]. Need AB, Davis RJ, Alexander-Chacko JT, et al. The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats. Psychopharmacology, 2006, 184(1): 26-35.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 487.4 |
| Cas No. | 362519-49-1 |
| Formula | C23H20Cl2N4O2S |
| Solubility | ≥47.7 mg/mL in DMSO; ≥4.85 mg/mL in EtOH with ultrasonic; insoluble in H2O |
| Chemical Name | (S,E)-3-(4-chlorophenyl)-N-((4-chlorophenyl)sulfonyl)-N'-methyl-4-phenyl-4,5-dihydro-1H-pyrazole-1-carboximidamide |
| SDF | Download SDF |
| Canonical SMILES | C/N=C(/NS(c(cc1)ccc1Cl)(=O)=O)\N(C[C@@H]1c2ccccc2)N=C1c(cc1)ccc1Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure