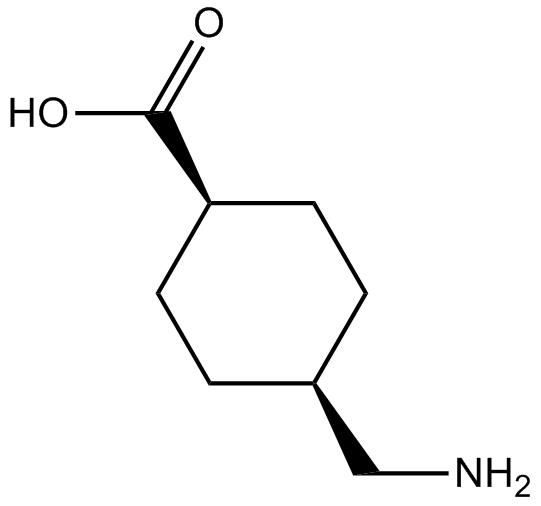
Tranexamic Acid
Tranexamic Acid is an antifibrinolytic, blocking lysine-binding sites (LBS) of plasmin and elastase-derived plasminogen fragments, with IC50 value of 5 mM [1].
Plasmin, generated from plasminogen under a reaction catalyzed by urokinase and tissue plasminogen activator, has a well-defined role in fibrinolysis. The LBS on plasmin and plasminogen mediate binding to fibrin and to cell surfaces, thereby enhancing the activation rate of plasminogen [1].
At concentrations of 0, 1, 2, 4, 6, 8, 10 μM, Tranexamic Acid dose-dependently inhibited porcine plasmin-induced neutrophil adherence to endothelial cell monolayers, with IC50 value of 5 mM. The neutrophil adherence inducing effect of plasmin was abolished under the treatment of 10 mM Tranexamic Acid [1].
In a rat bleeding model, where 8 mg/kg/h tissue plasmin activator (tPA) was continuously infused to prolong bleeding time beyond control values, Tranexamic Acid was administered by continuous infusion of 30, 100 mg/kg/h up to a dose of 300 mg/kg/h. Tranexamic Acid at 30 mg/kg/h showed no effects on bleeding, but exhibited a significant reduction in the bleeding time at 100 mg/kg/h, with the greatest effect seen at the highest dose of 300 mg/kg/h [2].
References:
[1]. Lo S K, Ryan T J, Gilboa N, et al. Role of catalytic and lysine-binding sites in plasmin-induced neutrophil adherence to endothelium. Journal of Clinical Investigation, 1989, 84(3): 793-801.
[2]. Sperzel M, Huetter J. Evaluation of aprotinin and tranexamic acid in different in vitro and in vivo models of fibrinolysis, coagulation and thrombus formation. Journal of Thrombosis and Haemostasis, 2007, 5(10): 2113-2118.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 157.21 |
| Cas No. | 1197-18-8 |
| Formula | C8H15NO2 |
| Solubility | insoluble in EtOH; insoluble in DMSO; ≥6.6 mg/mL in H2O |
| Chemical Name | 4-(aminomethyl)cyclohexane-1-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | C1CC(CCC1CN)C(=O)O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure