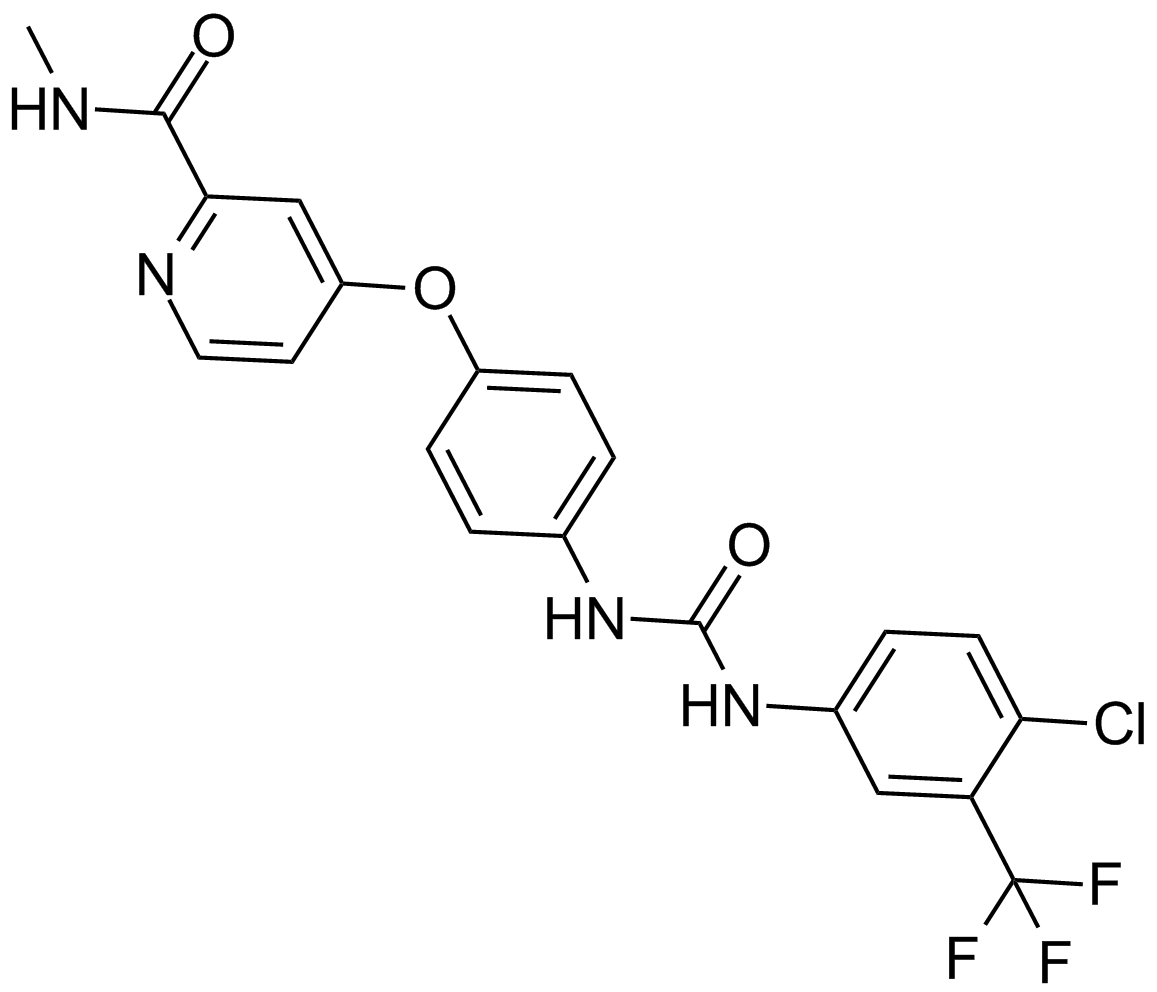
Sorafenib
Sorafenib (CAS 284461-73-0) is an orally bioavailable small molecule inhibitor targeting multiple kinases, including Raf kinase as well as receptor tyrosine kinases such as VEGFR2, PDGFR, FLT3, Ret, and c-Kit. By inhibiting Raf signaling pathways, sorafenib suppresses tumor cell proliferation, induces apoptosis, and disrupts tumor angiogenesis. In vitro studies demonstrate that sorafenib effectively inhibits Raf-mediated signaling cascades and blocks the proliferation of tumor cells. Sorafenib serves as an important research tool in the study of cancer biology, particularly in investigating antiangiogenic and antiproliferative mechanisms in various tumor models.
- 1. Fan Chen, Min-Hong Lv, et al. "Pexidartinib plus FLT3-directed CAR-Macrophage for the treatment of FLT3-ITD-mutated acute myeloid leukemia in preclinical model." bioRxiv. September 29, 2024.
- 2. Qi Wang, Nan Cheng, et al. "Synergistic Action of Benzyl Isothiocyanate and Sorafenib in a Nanoparticle Delivery System for Enhanced Triple-Negative Breast Cancer Treatment." Cancers (Basel). 2024 Apr 26;16(9):1695. PMID: 38730647
- 3. Yi-Jen Liao, et al. "Long-term di-(2-ethylhexyl) phthalate exposure reduces sorafenib treatment efficacy by enhancing mesenchymal transition in hepatocellular carcinoma." Ecotoxicol Environ Saf. 2024 Mar 15:273:116161. PMID: 38430581
- 4. Linzhuo Huang, Rui Xu, et al. "Repolarization of macrophages to improve sorafenib sensitivity for combination cancer therapy." Acta Biomater. 2023 May:162:98-109. PMID: 36931417
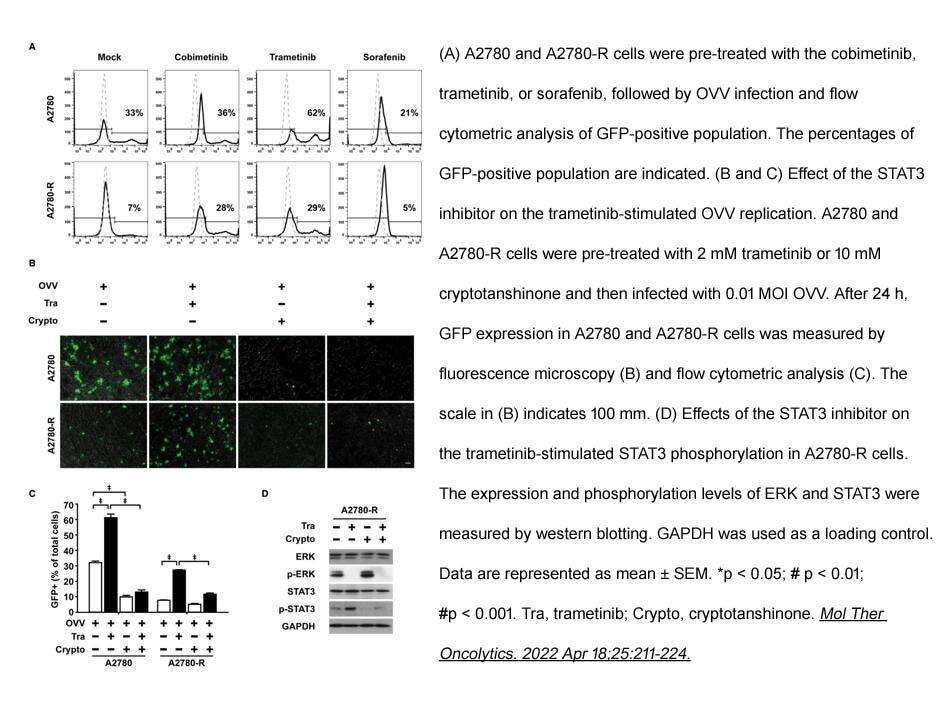
- 5. Seoyul Lee, Wookyeom Yang, et al. "Inhibition of MEK-ERK pathway enhances oncolytic vaccinia virus replication in doxorubicin-resistant ovarian cancer." Mol Ther Oncolytics. 2022 Apr 18;25:211-224. PMID: 35592390
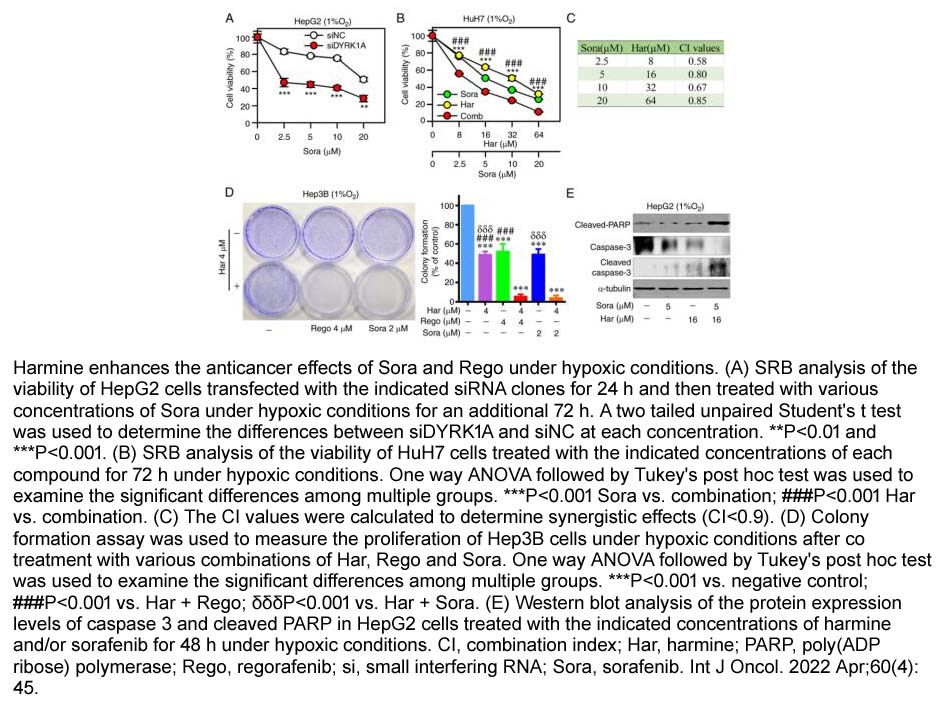
- 6. CHONG ZHANG, LIN‑WEN WU, et al. "DYRK1A suppression attenuates HIF‑1α accumulation and enhances the anti‑liver cancer effects of regorafenib and sorafenib under hypoxic conditions." Int J Oncol. 2022 Apr;60(4):45. PMID: 35244188
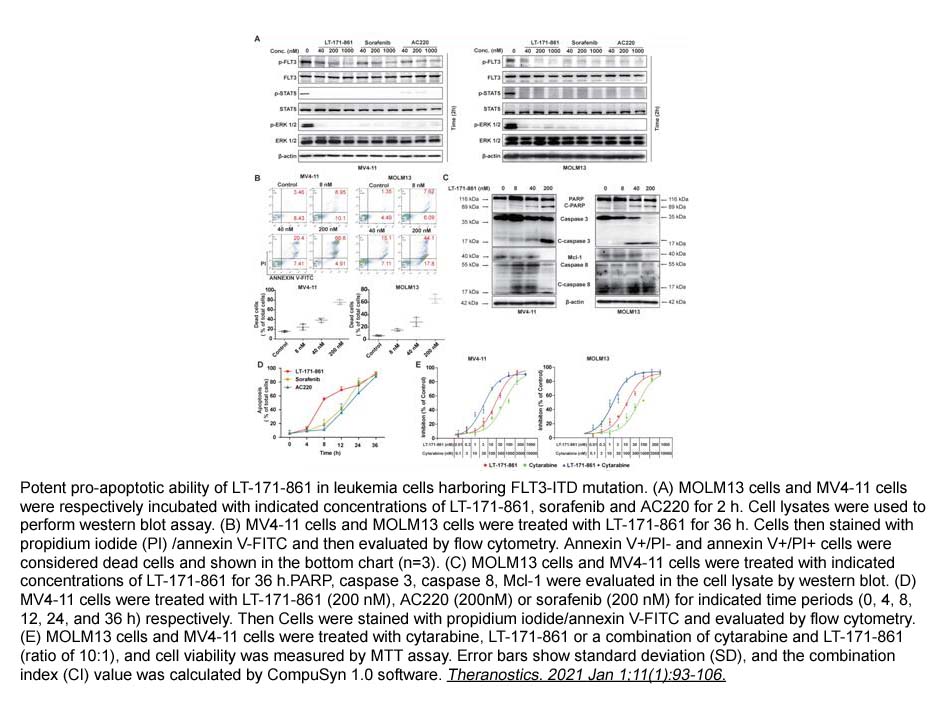
- 7. Zhou Yu, Jiaying Du, et al. "LT-171-861, a novel FLT3 inhibitor, shows excellent preclinical efficacy for the treatment of FLT3 mutant acute myeloid leukemia." Theranostics. 2021 Jan 1;11(1):93-106. PMID: 33391463
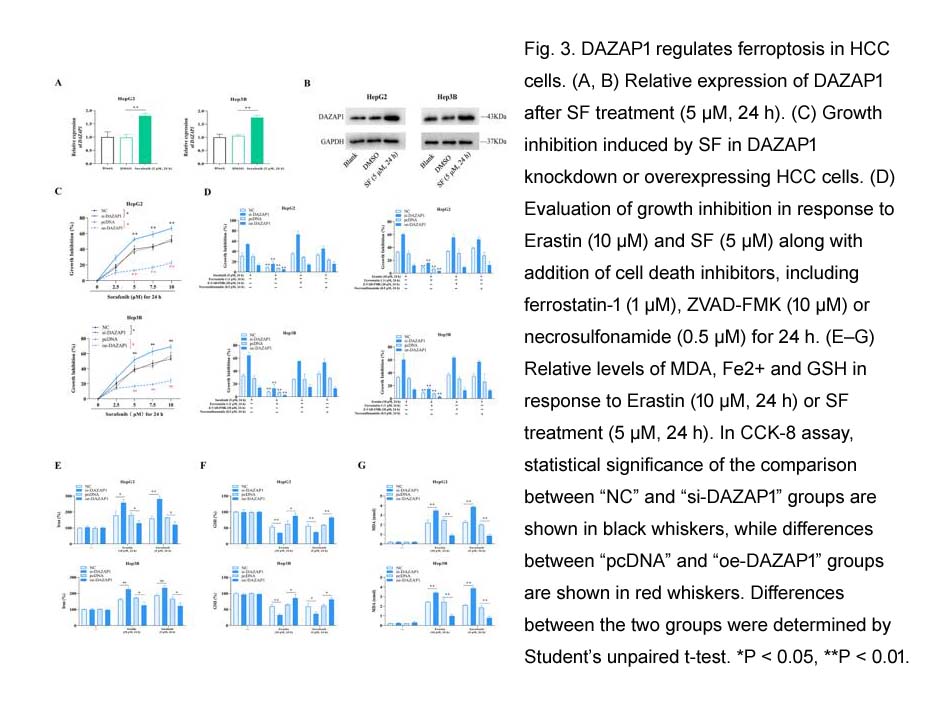
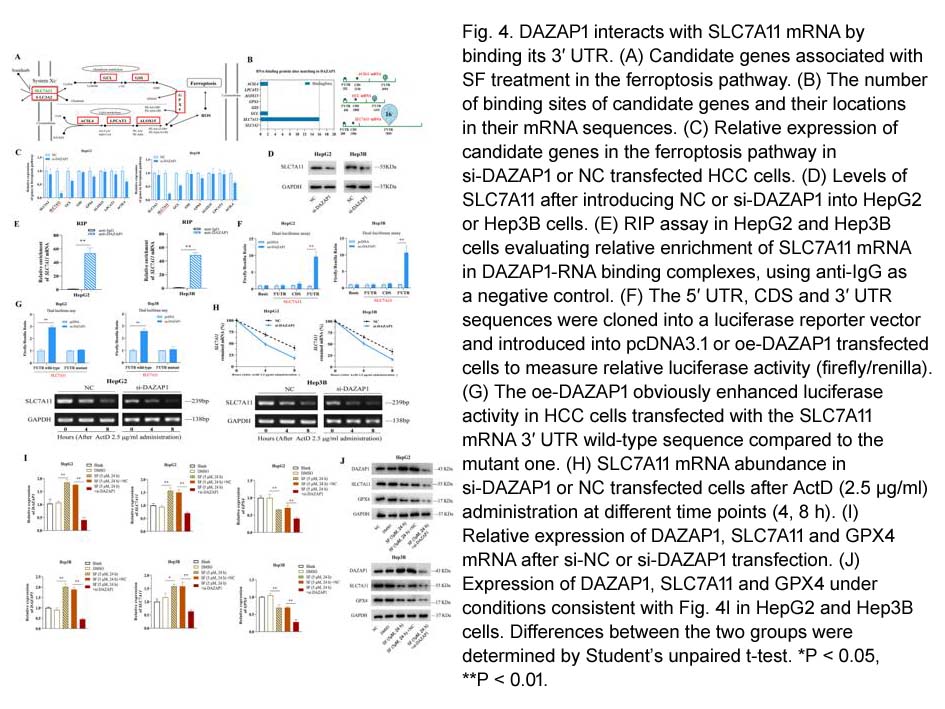
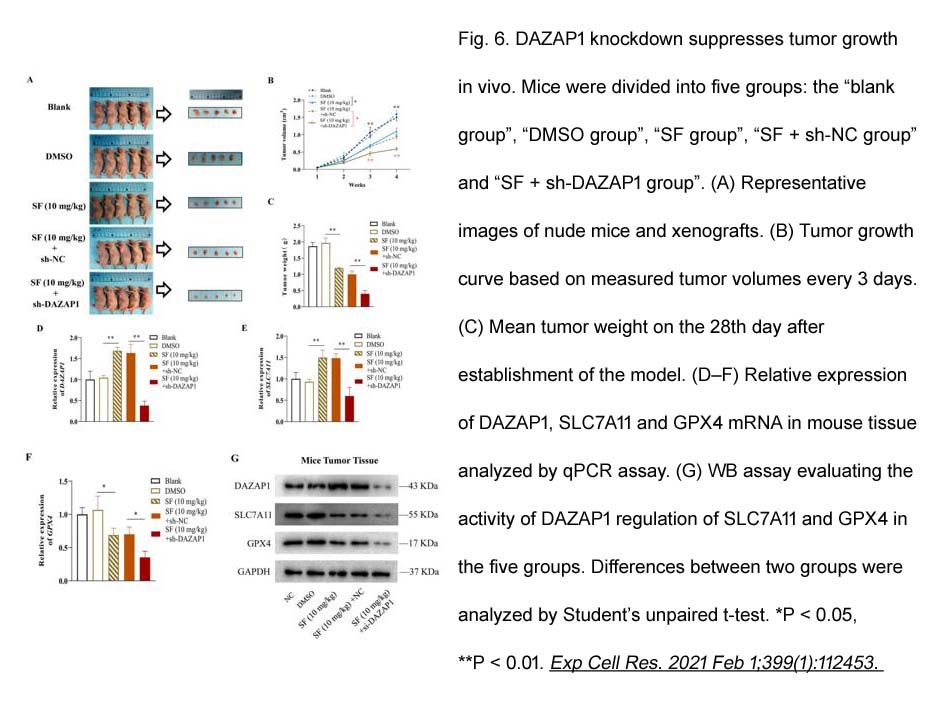
- 8. Qi Wang, Yaxun Guo, et al. "RNA binding protein DAZAP1 promotes HCC progression and regulates ferroptosis by interacting with SLC7A11 mRNA." Exp Cell Res. 2021 Feb 1;399(1):112453. PMID: 33358859
- 9. Bai J, Liu Z, et al. "Mitochondrial metabolic study guided by proteomics analysis in hepatocellular carcinoma cells surviving long-term incubation with the highest dose of sorafenib." Aging (Albany NY), 11 (24), 12452-12475 2019 Dec 26. PMID: 31881007
- 10. Cheriyan VT, Alsaab H, et al. "A CARP-1 functional mimetic compound is synergistic with BRAF-targeting in non-small cell lung cancers." Oncotarget. 2018 Jul 3;9(51):29680-29697. PMID: 30038713
- 11. Sieber J, Wieder N, et al. "GDC-0879, a BRAF(V600E) Inhibitor, Protects Kidney Podocytes from Death." Cell Chem Biol. 2017 Dec 6. PMID: 29249695
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 464.82 |
| Cas No. | 284461-73-0 |
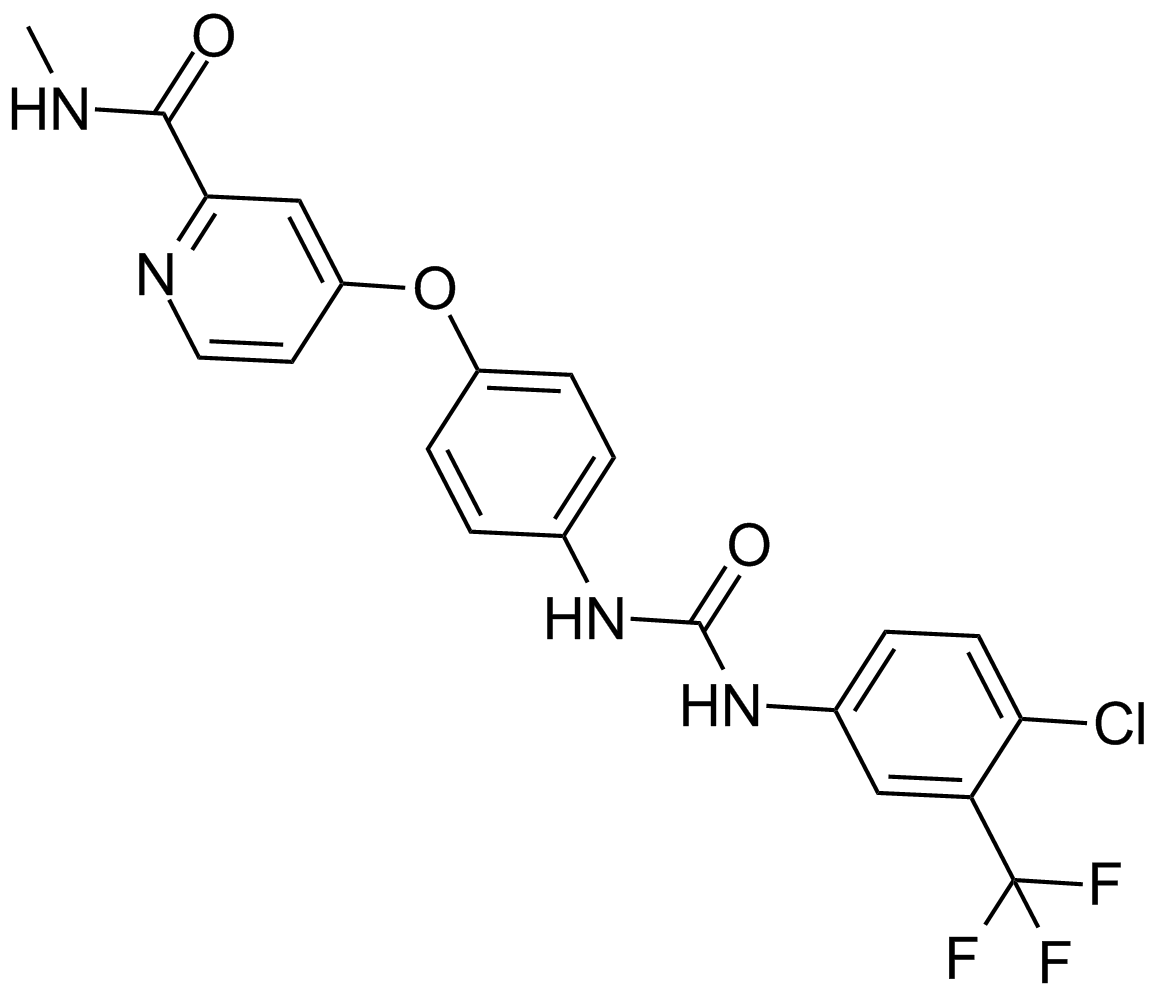
| Formula | C21H16ClF3N4O3 |
| Synonyms | BAY-43-9006, Sorafenib, Nexavar, sorafenibum |
| Solubility | ≥23.25 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
| Chemical Name | 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-methylpyridine-2-carboxamide |
| SDF | Download SDF |
| Canonical SMILES | CNC(c1nccc(Oc(cc2)ccc2NC(Nc(cc2)cc(C(F)(F)F)c2Cl)=O)c1)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment: [1] | |
|
Cell lines |
PLC/PRF/5 and HepG2 cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
IC50: 6.3 μM for PLC/PRF/5 cells 4.5 μM for HepG2 cells 72 hours |
|
Applications |
The effect of sorafenib on cell proliferation was measured by CellTiter-Glo assay. Sorafenib inhibited cell proliferation dose-dependently with an IC50 of 6.3 μmol/L in PLC/PRF/5 and 4.5 μmol/L in HepG2 cells. |
| Animal experiment: [1] | |
|
Animal models |
Female CB17 SCID mice injected with PLC/PRF/5 cells |
|
Dosage form |
Oral administration; 10, 30, and 100 mg/kg body weight; once daily for 16 or 21 days |
|
Applications |
Sorafenib tosylate produced dose-dependent growth inhibition of s.c. implanted PLC/PRF/5 tumor xenografts in SCID mice. Dose levels of 10 and 30 mg/kg produced significant and dose-dependent TGIs of 49% and 78%, respectively. Sorafenib tosylate produced durable partial tumor regressions in 50% of the mice at the 100 mg/kg dose level. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer research, 2006, 66 (24): 11851-11858. |
|
| Description | Sorafenib is a multikinase inhibitor of Raf-1, B-Raf and VEGFR-2 with IC50 of 6 nM, 22 nM and 90 nM, respectively. | |||||
| Targets | B-Raf | VEGFR2 | PDGFRβ | |||
| IC50 | 6 nM | 22 nM | 90 nM | 57 nM | ||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data

Related Biological Data
Related Biological Data