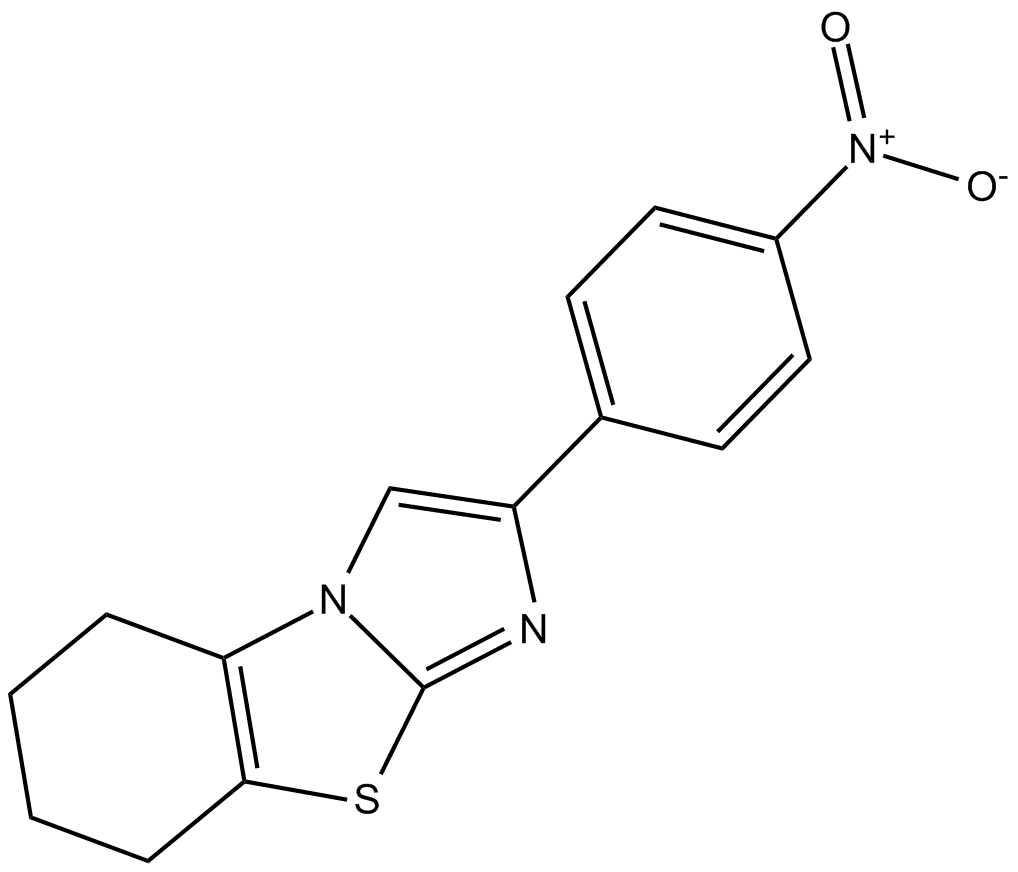
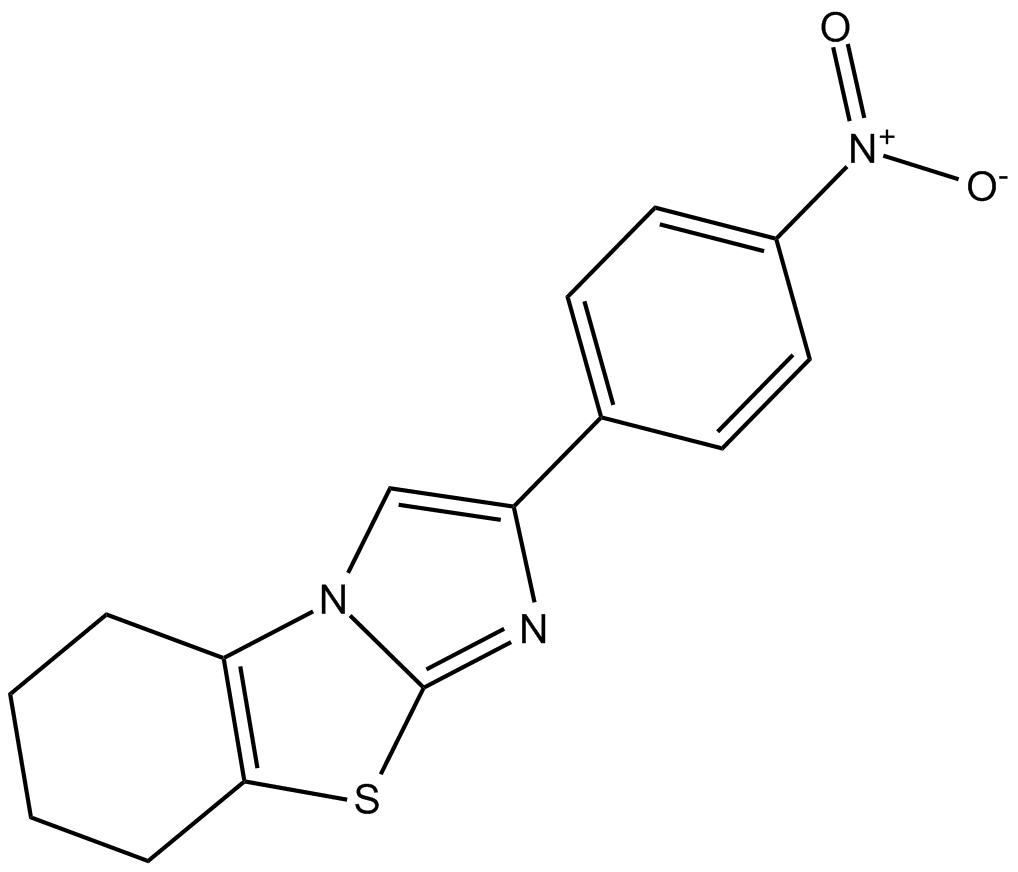
p-nitro-Cyclic Pifithrin-α
p-nitro-Cyclic Pifithrin-α is an inactivator of p53.
The activation of the tumor suppressor gene p53 plays a key role in regulating the in-vitro death of neurons, following apoptotic stimuli molecules including glutamate and DNA-damaging agents. Thus, p53 inhibitors may prove effective in suppressing the degenerative processes in neurodegenerative disorders.
In vitro: Pifithrin-α (PFT-α) was identified as an inactivator of p53 blocking p53-dependent transcriptional activation and apoptosis. Cyclic PFT-α was a stable analog of PFT-α. p-nitro-Cyclic PFT-α, a cell-permeable form of cyclic PFT-α, was found to be one order of magnitude more active than PFT-α in protecting cortical neurons exposed to etoposide. p-nitro-Cyclic PFT-α acted in a p53-dependently but did not block phosphorylation of p53 on Ser15 in response to etoposide treatment, although it prevented p53 posttranscriptional activity [1].
In vivo: In a previou study, C57BL/6 mice were fed a high-fat (HFD) or control diet for 8 weeks; PFT was administered three times per week. Results showed that PFT administration could suppress HFD-induced weight gain, steatosis, oxidative stress, ALT elevation, and apoptosis. PFT treatment also able to blunt the HFD-induced upregulation of miRNA34a and increase SIRT1 expression. In the livers of HFD-fed, PFT-treated mice, activation of the SIRT1/PGC1α/PPARα axis increased the expression of malonyl-CoA decarboxylase [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] . Pietrancosta, N.,Moumen, A.,Dono, R., et al. Imino-tetrahydro-benzothiazole derivatives as p53 inhibitors: Discovery of a highly potent in vivo inhibitor and its action mechanism. Journal of Medicinal Chemistry 49(12), 3645-3652 (2006).
[2] Derdak Z, Villegas KA, Harb R, Wu AM, Sousa A, Wands JR. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. J Hepatol. 2013 Apr;58(4):785-91.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 299.3 |
| Cas No. | 60477-38-5 |
| Formula | C15H13N3O2S |
| Synonyms | Cyclic pifithrin-α-p-nitro,p-nitro-Cyclic PFT-α |
| Solubility | ≤1mg/ml in dimethyl formamide |
| Chemical Name | 5,6,7,8-tetrahydro-2-(4-nitrophenyl)-imidazo[2,1-b]benzothiazole |
| SDF | Download SDF |
| Canonical SMILES | [O-][N+](c(cc1)ccc1-c1c[n](c(CCCC2)c2[s]2)c2n1)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity ≥ 95.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure