Necrosulfonamide
Necrosulfonamide (NSA) is a pharmacological inhibitor of mixed lineage kinase-like protein (MLKL) [1]. NSA is potent in protecting necrotic/necroptotic death of human HT-29 with an IC50 value of 124 nM [2].
MLKL, a functional RIP3 substrate, can bind to RIP3 through its kinase-like domain but it lacks kinase activity. MLKL can be phosphorylated by RIP3 at the T357 and S358 sites [3].
Treatment with NSA alone did not rescue cell death, while NSA significantly enhanced the protection of zVAD.fmk against BV6/5AC-induced cell death. In the same line, knockdown of MLKL did not significantly protect cells against BV6/5AC cotreatment in the absence of zVAD.fmk [1]. In the Dox-treated HeLa cells, NSA inhibited necrosis. With a higher level of RIP3, the allosteric inhibition of necrostatin-1 on RIP1 was overcome by cells. In contrast, NSA still efficiently prevented necrosis under this condition. Consistently, knockdown of MLKL also blocked necrosis. Under necrosis-inducing conditions, the presence of NSA made tubular mitochondrial morphology remain normal. Consistently the mitochondrial morphological changes were also prevented by the knockdown of MLKL [4]. Even at 5 μM concentration, NSA had no effect on the apoptosis induced by TNF-α plus Smac mimetic in non-RIP3-expressing Panc-1 cells. In the presence of NSA, the discrete RIP3 punctae were detected but failed to enlarge. That meant NSA blocked necrosis at a specific step in the necrosis pathway [3].
Pharmacological treatment with NSA delayed cone degeneration [5].
References:
[1]. Gerges S, Rohde K, Fulda S. Cotreatment with Smac mimetics and demethylating agents induces both apoptotic and necroptotic cell death pathways in acute lymphoblastic leukemia cells[J]. Cancer letters, 2016, 375(1): 127-132.
[2]. Bae JH, Shim JH, Cho YS. Chemical regulation of signaling pathways to programmed necrosis[J]. Archives of pharmacal research, 2014, 37(6): 689-697.
[3]. Wang H, Sun L, Su L, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3[J]. Molecular cell, 2014, 54(1): 133-146.
[4]. Wang Z, Jiang H, Chen S, et al. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways[J]. Cell, 2012, 148(1): 228-243.
[5]. Viringipurampeer IA, Mohammadi Z, Shan X, et al. Rip3 knockdown rescues photoreceptor cell death in pde6c zebrafish model of achromatopsia[J]. Investigative Ophthalmology & Visual Science, 2013, 54(15): 5955-5955.
- 1. Fubin Zhang, Tianhong Zhu, et al. "FTO triggers NLRP3/GSDMD - dependent pyroptosis to enhance cisplatin - sensitivity in ovarian cancer." Volume 131, July 2025, 111698
- 2. Rafaela Rosa-Ribeiro, Mateus Lucas Falco, et al. "Unraveling Zika virus-induced cell death pathways in eradicating embryonal central nervous system tumors." Neuroscience. 2025 Jul 23:582:126-133 PMID: 40712830
- 3. Xiaoming Liu, Haipeng Liu, et al. "The interactive toxic effect of homocysteine and copper on cardiac microvascular endothelial cells during ischemia-reperfusion injury." Chem Biol Interact. 2025 Jan15:408:111387 PMID: 39824432
- 4. Haipeng Liu, Siyang Yu, Shansong Gao. "Peroxynitrite regulates ER stress-mediated Ca2+ flux to mitochondria characterizing cardiac microvascular ischemia–reperfusion injury associated with hyperhomocysteinemia." J Transl Med. 2025 Nov 10;23(1):1254 PMID: 41214689
- 5. Zining Wang, Lei Cui, et al. "Cancer cell-intrinsic biosynthesis of itaconate promotes tumor immunogenicity." EMBO J. 2024 Sep 30 PMID: 39349845
- 6. Xurui Li, Wei Xiong, et al. "p53 activates the lipoxygenase activity of ALOX15B via inhibiting SLC7A11 to induce ferroptosis in bladder cancer cells." Lab Invest. 2023 May;103(5):100058 PMID: 36801644
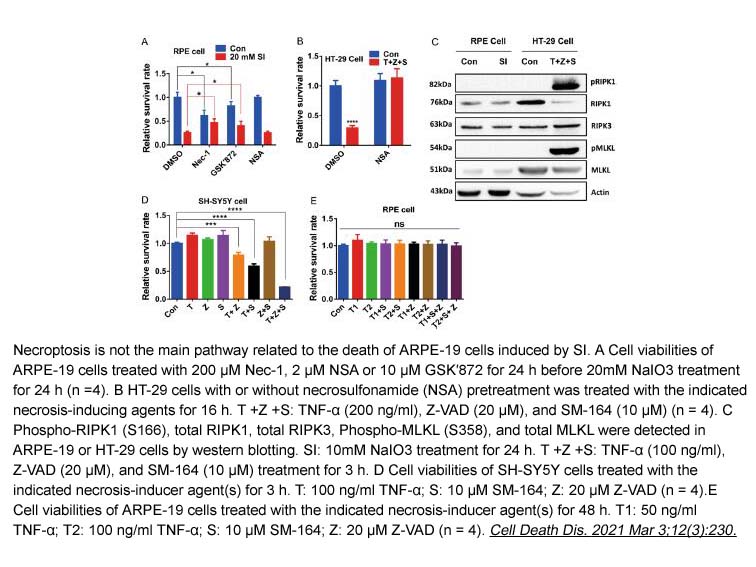
- 7. Binghua Liu, Weiyan Wang, et al. "Sodium iodate induces ferroptosis in human retinal pigment epithelium ARPE-19 cells." Cell Death Dis. 2021 Mar 3;12(3):230 PMID: 33658488
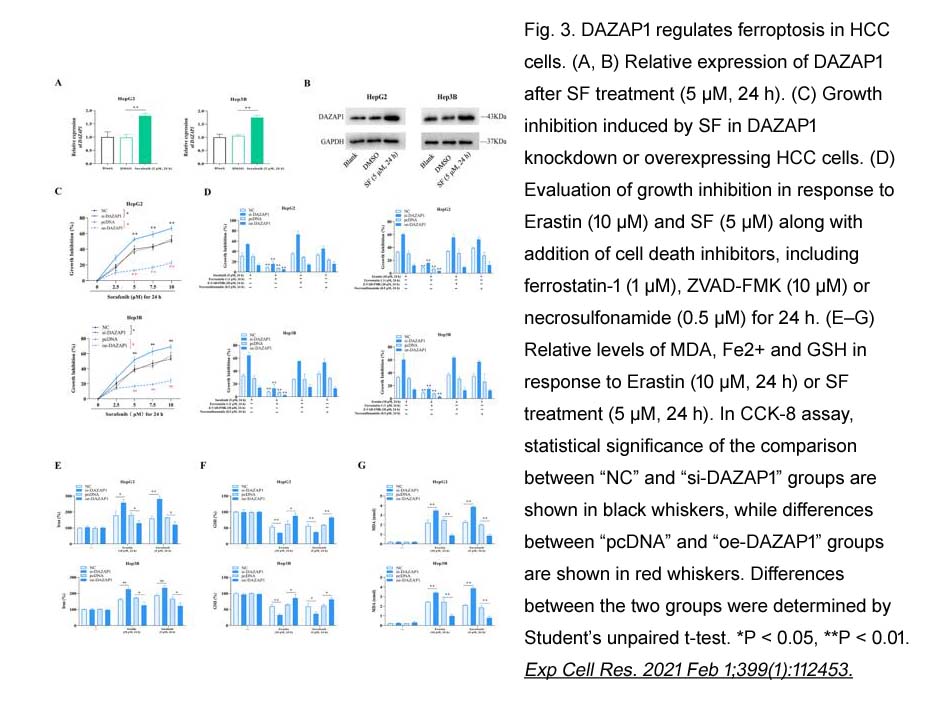
- 8. Qi Wang, Yaxun Guo, et al. "RNA binding protein DAZAP1 promotes HCC progression and regulates ferroptosis by interacting with SLC7A11 mRNA." Exp Cell Res. 2021 Feb 1;399(1):112453 PMID: 33358859
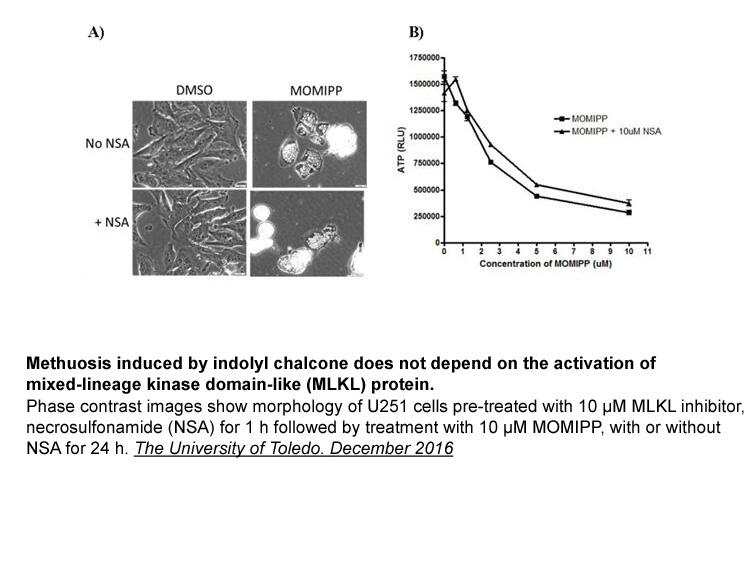
- 9. Nneka Elizabeth Mbah. "Defining the Mechanism of Methuosis, a Non-apoptotic Cell Death Pathway, Induced by Indolyl Chalcone Compounds in Glioblastoma Cells." The University of Toledo.December 2016
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 461.47 |
| Cas No. | 1360614-48-7 |
| Formula | C18H15N5O6S2 |
| Solubility | ≥46.1 mg/mL in DMSO; insoluble in EtOH; insoluble in H2O |
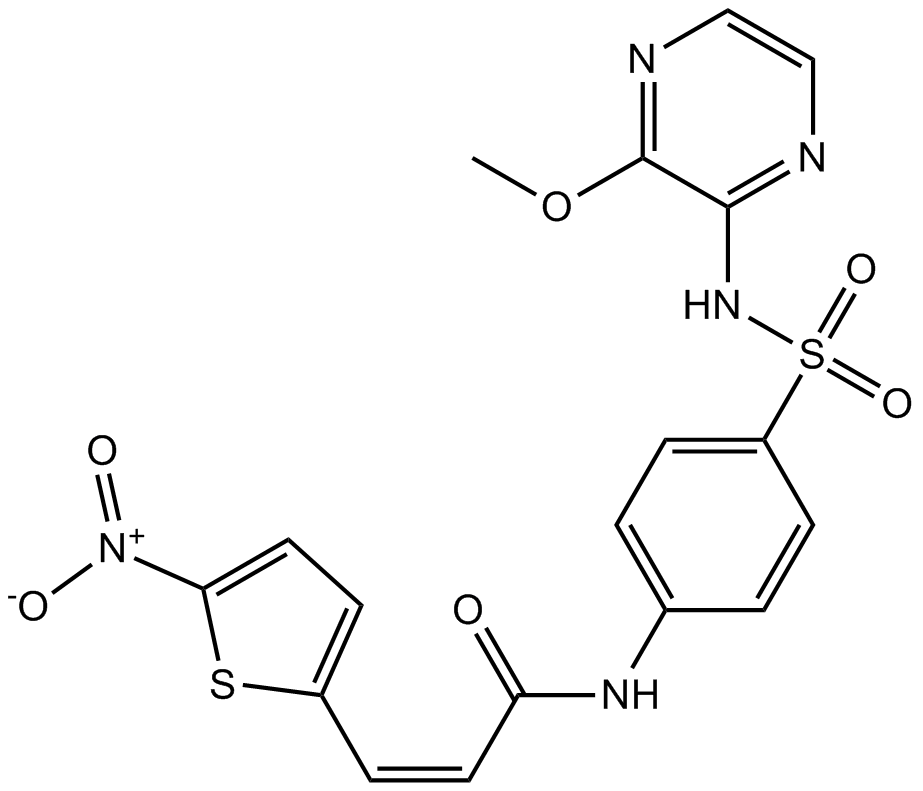
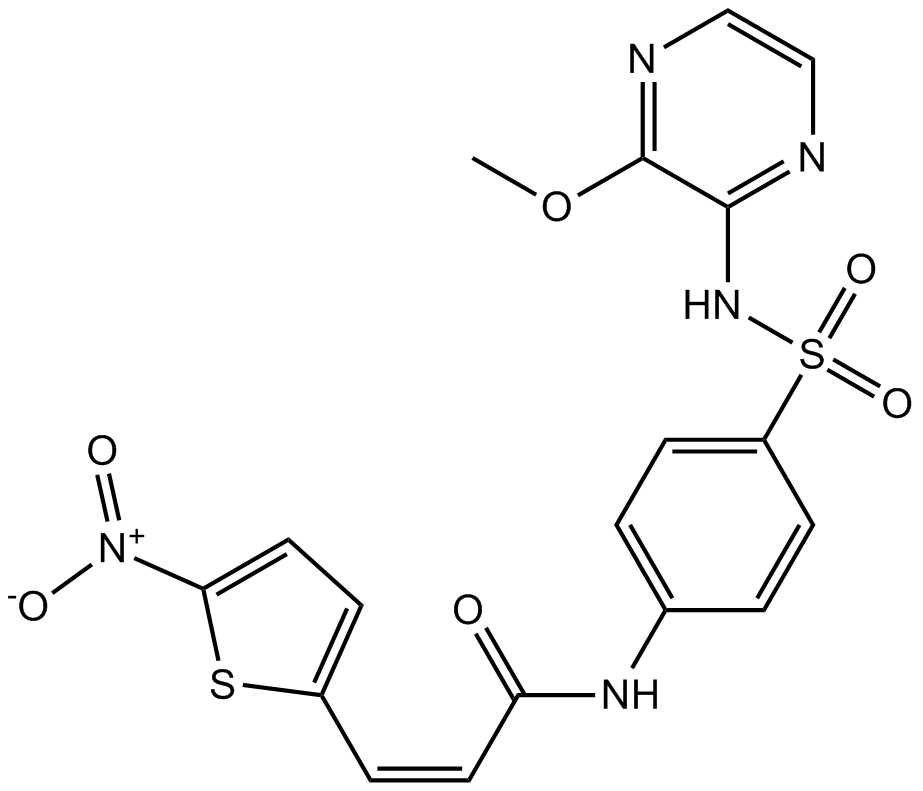
| Chemical Name | (Z)-N-(4-(N-(3-methoxypyrazin-2-yl)sulfamoyl)phenyl)-3-(5-nitrothiophen-2-yl)acrylamide |
| SDF | Download SDF |
| Canonical SMILES | COc1nccnc1NS(c(cc1)ccc1NC(/C=C\c1ccc([N+]([O-])=O)[s]1)=O)(=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[1] | |
|
Cell lines |
Human colorectal cancer HT-29 cells |
|
Reaction Conditions |
1 μM necrosulfonamide for 8 or 12 h incubation |
|
Applications |
Necrosulfonamide treatment (1 μM; 8 or 12 h incubation) completely blocked necroptosis by disturbing MLKL-induced liposome leakage in HT-29 cells treated with T/S/Z. Although necrosulfonamide did not prevent MLKL phosphorylation, it was able to block p-MLKL translocation to the membrane fraction in HT-29 cells subjected to T/S/Z treatment. Necrosulfonamide was used to explore the role of MLKL in membrane integrity and necrotic death. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Wang H, Sun L, Su L, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Molecular Cell, 2014, 54(1): 133-146. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data