MK 886
MK 886 is a potent, cell-permeable and orally-active inhibitor of 5-lipoxygenase-activating protein (FLAP), with an IC50 value of 30 nM for inhibition of [125I]-L-691,678 photoaffinity labelling. FLAP is essential for the activation of 5-lipoxygenase (5-LO) and therefore for the biosynthesis of leukotrienes. Leukotrienes, the biologically active metabolites of arachidonic acid, have been implicated in various inflammatory responses, such as asthma, arthritis as well as psoriasis. In addition, MK 886 is also a non-competitive antagonist of the peroxisome-proliferator-activated receptor alpha (PPARα), with the ability to induce apoptosis.
References:
1. Mancini JA, Prasit P, Coppolino MG, et al. 5-Lipoxygenase-activating protein is the target of a novel hybrid of two classes of leukotriene biosynthesis inhibitors. Molecular Pharmacology, 1992, 41(2): 267-272.
2. Dixon RA, Diehl RE, Opas E, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature, 1990, 343(6255): 282-284.
3. Kehrer JP, Biswal SS, La E, et al. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochemical Journal, 2001, 356(Pt 3): 899-906.
4. Imbesi M, Zavoreo I, Uz T, et al. 5-Lipoxygenase inhibitor MK-886 increases GluR1 phosphorylation in neuronal cultures in vitro and in the mouse cortex in vivo. Brain Research, 2007, 1147: 148-153.
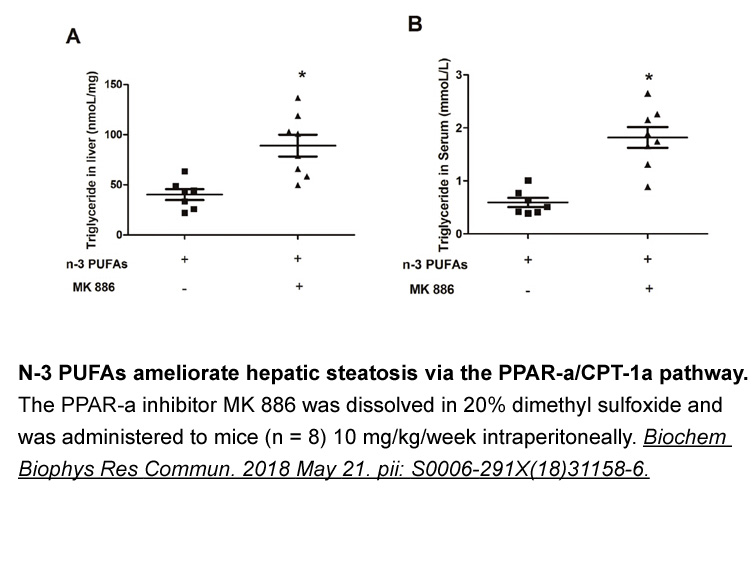
- 1. Yingfen Dai, Zhimeng Lv, et al. "PPARα alleviates inflammation via inhibiting NF-κB/Rel pathway in Vibrio splendidus challenged Apostichopus japonicus." Fish Shellfish Immunol. 2023 Apr:135:108701. PMID: 36948368 2. Tian F, Wang J, et al."N-3 polyunsaturated fatty acids ameliorate hepatic steatosis via the PPAR-α/CPT-1α pathway in a mouse model of parenteral nutrition." Biochem Biophys Res Commun. 2018 Jul 2;501(4):974-981. PMID:29777706
| Physical Appearance | White solid |
| Storage | Store at RT |
| M.Wt | 472.08 |
| Cas No. | 118414-82-7 |
| Formula | C27H34ClNO2S |
| Solubility | ≥15.1 mg/mL in DMSO with ultrasonic; ≥2.16 mg/mL in EtOH with ultrasonic; insoluble in H2O |
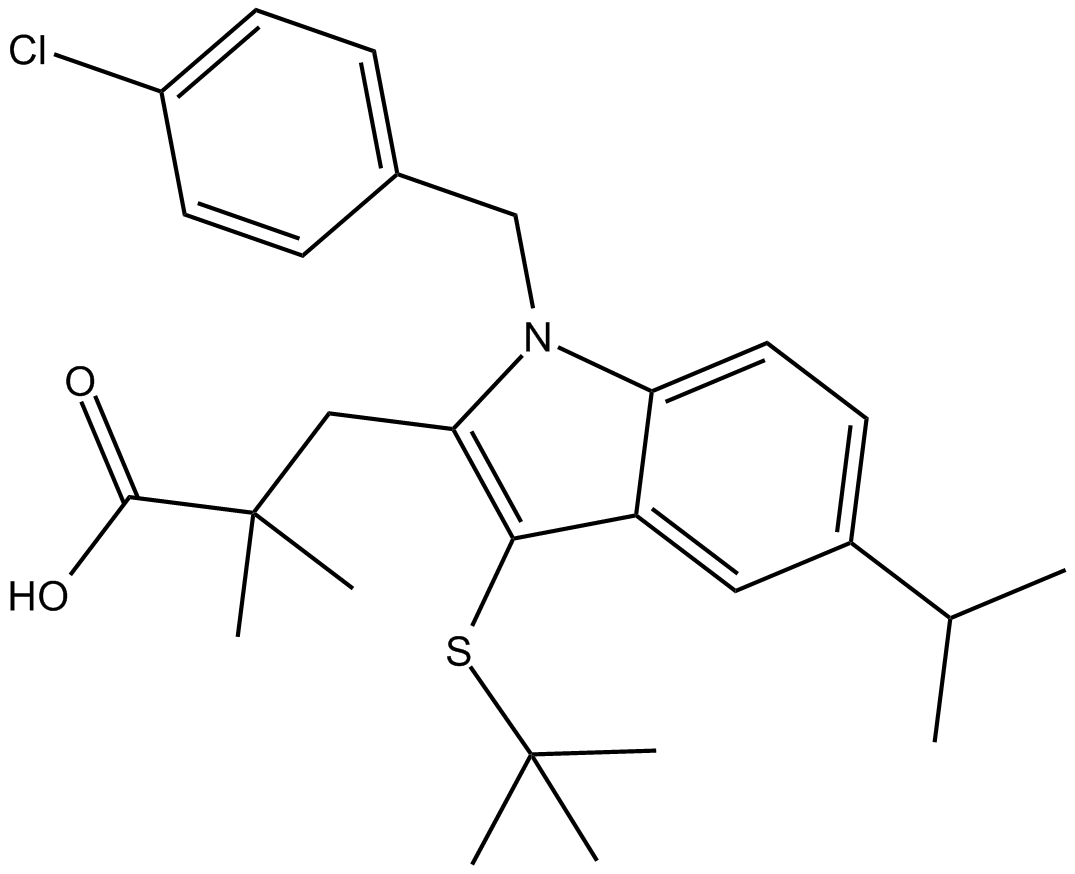
| Chemical Name | 3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-isopropyl-1H-indol-2-yl)-2,2-dimethylpropanoic acid |
| SDF | Download SDF |
| Canonical SMILES | ClC1=CC=C(C=C1)CN(C2=CC=C(C(C)C)C=C32)C(CC(C)(C(O)=O)C)=C3SC(C)(C)C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[2] | |
|
Cell lines |
Osteosarcoma cells expressing 5-LO, 5-LO and rat FLAP (5-LO/FLAP), or rat neutrophils |
|
Reaction Conditions |
0.3 μM MK 886 for osteosarcoma cells and 0.2 μM MK 886 for rat neutrophils |
|
Applications |
MK 886 blocked leukotriene synthesis both in the 5-LO/FLAP cell line and in neutrophils. MK 886 was able to inhibit the synthesis of leukotrienes in intact activated leukocytes, but showed little or no effect on enzymes directly involved in leukotriene synthesis, including 5-LO. |
| Animal experiment:[4] | |
|
Animal models |
Male C57BL/6J mice |
|
Dosage form |
3 mg/kg Intraperitoneal (i.p.) injection |
|
Applications |
Repeated daily i.p. injections of MK 886 increased phosphorylation of AMPAR subunit glutamate receptor 1 (GluR1) in brain samples obtained from the prefrontal cortex, whereas a single injection of MK 886 did not alter cortical GluR1 phosphorylation. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Mancini JA, Prasit P, Coppolino MG, et al. 5-Lipoxygenase-activating protein is the target of a novel hybrid of two classes of leukotriene biosynthesis inhibitors. Molecular Pharmacology, 1992, 41(2): 267-272. 2. Dixon RA, Diehl RE, Opas E, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature, 1990, 343(6255): 282-284. 3. Kehrer JP, Biswal SS, La E, et al. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochemical Journal, 2001, 356(Pt 3): 899-906. 4. Imbesi M, Zavoreo I, Uz T, et al. 5-Lipoxygenase inhibitor MK-886 increases GluR1 phosphorylation in neuronal cultures in vitro and in the mouse cortex in vivo. Brain Research, 2007, 1147: 148-153. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data