LY2606368
LY2606368 is a selective ATP competitive inhibitor of checkpoint kinase 1(CHK1) with IC50 value of 1.5nM in SW1990 cells [1].
CHK1 is an intracellular serine/threonine kinase that plays a role in DNA damage response pathway. The inhibitors of CHK1 are developed for the treatment of cancers. LY2606368 is an ATP-competitive inhibitor of CHK1 and is undergoing clinical trials currently. It inhibits the auto-phosphorylation of CHK1 and induces the phosphorylation of H2AX in cancer cells. In the pancreatic cell line SW1990, LY2606368 significantly inhibits cell proliferation with IC50 value of 1.5nM. LY2606368 also exerts potent anti-tumor activity in SW1990 xenograft model. Besides that, in the orthotopic SKVO3 model, treatment of LY2606368 is found to inhibit tumor growth and reduce the incidence of metastases and accumulation. However, LY2606368 is only administered intravenously due to its poor oral bioavailability [1, 2 and 3].
References:
[1] Wu W, Bi C, Bence A K, et al. Antitumor activity of Chk1 inhibitor LY2606368 as a single agent in SW1990 human pancreas orthotopic tumor model. Cancer Research, 2012, 72(8 Supplement): 1776.
[2] Lainchbury M, Matthews T P, McHardy T, et al. Discovery of 3-alkoxyamino-5-(pyridin-2-ylamino) pyrazine-2-carbonitriles as selective, orally bioavailable CHK1 inhibitors. Journal of medicinal chemistry, 2012, 55(22): 10229-10240.
[3] McNeely S C, Burke T F, DurlandBusbice S, et al. Abstract A108: LY2606368, a second generation Chk1 inhibitor, inhibits growth of ovarian carcinoma xenografts either as monotherapy or in combination with standard-of-care agents. Molecular Cancer Therapeutics, 2011, 10(Supplement 1): A108.
| Storage | Store at -20°C |
| M.Wt | 365.39 |
| Cas No. | 1234015-52-1 |
| Formula | C18H19N7O2 |
| Synonyms | Prexasertib |
| Solubility | insoluble in DMSO |
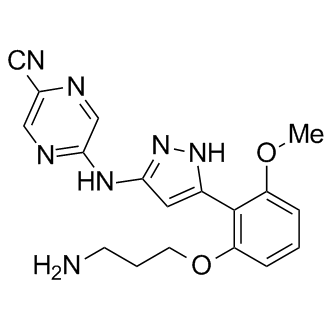
| Chemical Name | 5-((5-(2-(3-aminopropoxy)-6-methoxyphenyl)-1H-pyrazol-3-yl)amino)pyrazine-2-carbonitrile |
| SDF | Download SDF |
| Canonical SMILES | COc1cccc(OCCCN)c1-c1cc(Nc2ncc(C#N)nc2)n[nH]1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Hela cells |
|
Preparation method |
Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reaction Conditions |
7 h |
|
Applications |
LY2606368 triggers DNA damage during S-phase as pH2AX (S139) and TUNEL-positive staining cells increases substantially in Sphase cells. LY2606368 also need CDC25A and CDK2 to trigger DNA damage. In addition, LY2606368 leads to replication catastrophe. |
| Animal experiment [1]: | |
|
Animal models |
Female CD-1 nu-/nu- mice (26–28 g) bearing Calu-6 tumor |
|
Dosage form |
Twice daily for 3 days with 1, 3.3, or 10 mg/kg of LY2606368 |
|
Applications |
Up to 72.3% tumor growth inhibition is observed in all three doses of LY2606368 groups. Wight loss of mice is not exceeded by 3%, indicating the LY2606368 is well tolerated in any of the treatment groups. Moreover, tumor regrowth of the highest dose group is slow during the 28-day recovery period, suggesting a durable response to LY2606368. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: 1. King C, Diaz HB, McNeely S et al. LY2606368 Causes Replication Catastrophe and Antitumor Effects through CHK1-Dependent Mechanisms. Mol Cancer Ther. 2015 Sep;14(9):2004-13. |
|
Quality Control & MSDS
- View current batch:
Chemical structure