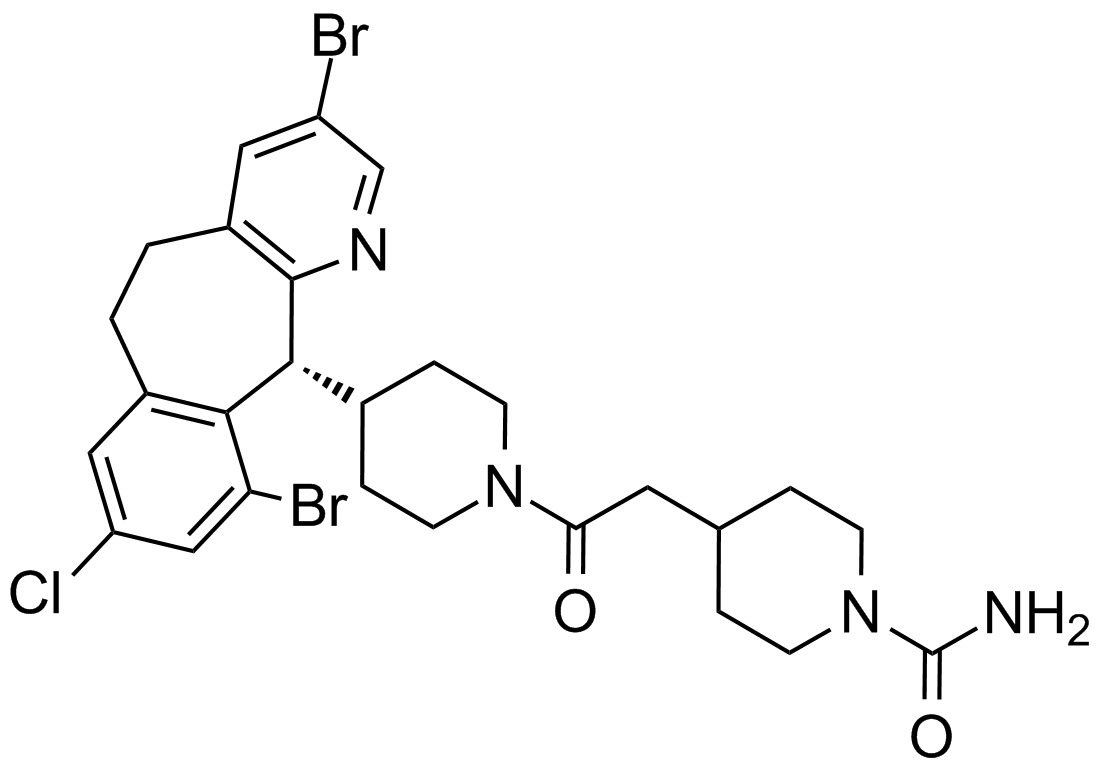
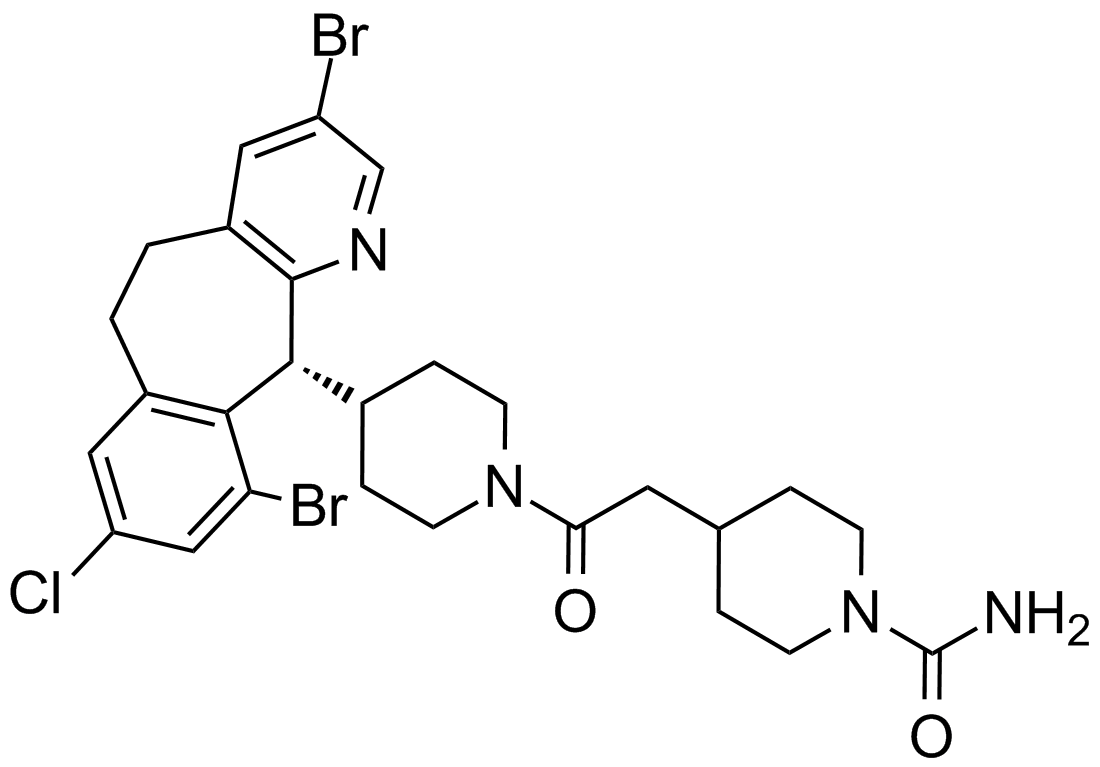
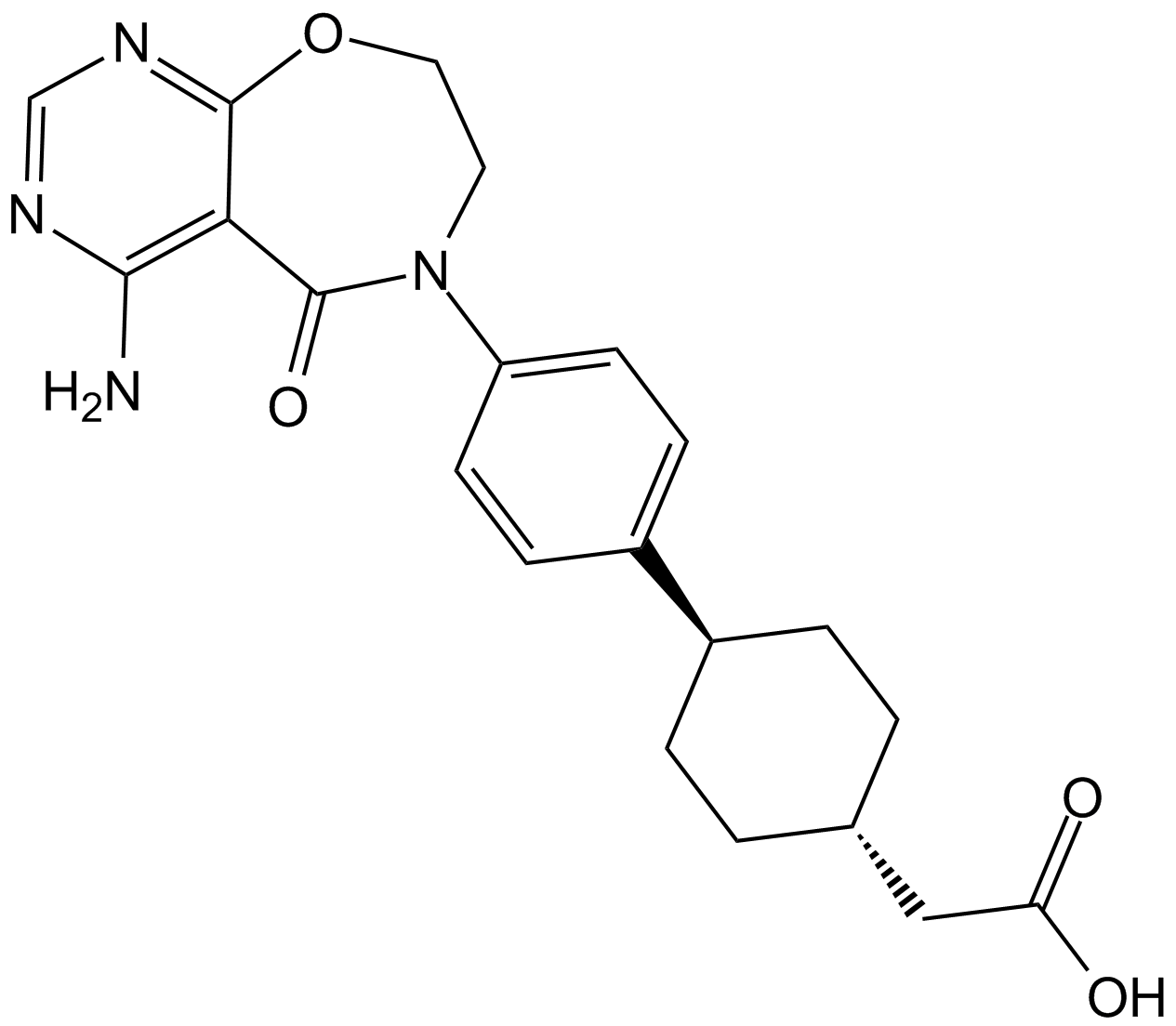
Lonafarnib
Lonafarnib (SCH66336, Sarasar) is an potent, selective, orally, bioavailable tricyclic nonpeptidyl nonsulfhydry inhibitor of farnesyltransferase (FTase).[1] It is a small molecular with the formula of C27H31Br2ClN4O2 and molecular weight of 638.82. Farnesylated Ras proteins was found to regulate signal transduction pathways which drive cell proliferation, growth and survival and be required for its membrane localization.[1, 2] Lonafarnib inhibits the post-translational farnesylcation of ras proteins, therefore blocking translocation of RAS to the plasma membrane.[3]
Reference
[1] Eric W, Malcolm J. M, Kim N. C, D. Scott E, et al. A multinomial Phase II study of lonafarnib (SCH 66336) in patients with refractory urothelial cancer. Urologic Oncology: Seminars and Original Investigations. 2005, 23. 143-149.
[2] Gongjie L, Stacey A. T, Cindy H. M, Yunsheng H, W. Robert B, et al. Continuous and intermittent dosing of lonafarnib potentiates the therapeutic efficacy of docetaxel on preclinical human prostate cancer models. Int. J. Cancer. 2009, 125. 2711–2720.
[3] Vasiliki A. N, Alexander J. S, Keith T. F, Hensin T, et al. Melanoma: New Insights and New Therapies. J Invest Dermatol. 2012, 132. 854–863.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 638.82 |
| Cas No. | 193275-84-2 |
| Formula | C27H31Br2ClN4O2 |
| Synonyms | Sch 66336, Sch66336, Sch-66336 |
| Solubility | ≥31.95 mg/mL in DMSO; insoluble in H2O; ≥96.4 mg/mL in EtOH with ultrasonic |
| Chemical Name | 4-[2-[4-[(11R)-3,10-dibromo-8-chloro-6,11-dihydro-5H-benzo[1,2]cyclohepta[2,4-b]pyridin-11-yl]piperidin-1-yl]-2-oxoethyl]piperidine-1-carboxamide |
| SDF | Download SDF |
| Canonical SMILES | C1CN(CCC1CC(=O)N2CCC(CC2)C3C4=C(C=C(C=C4CCC5=CC(=CN=C35)Br)Cl)Br)C(=O)N |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
UMSCC10B, UMSCC14B, UMSCC17B, UMSCC22B, UMSCC35 and UMSCC38 cells |
|
Preparation method |
The solubility of this compound in DMSO is > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20 °C for several months. |
|
Reaction Conditions |
0.1 ~ 8 μM; 24 hrs |
|
Applications |
In human head and neck squamous carcinoma cells (HNSCCs), SCH66336 (0.1 ~ 8 μM) suppressed cell growth and induced apoptosis of in a dose- and time- dependent manner. |
| Animal experiment [2]: | |
|
Animal models |
NOD/SCID mice bearing XEN08 tumors |
|
Dosage form |
50 mg/kg; p.o.; b.i.d., for 20 days |
|
Applications |
In NOD/SCID mice bearing XEN08 tumors, SCH66336 (50 mg/kg, p.o., b.i.d.) significantly inhibited tumor growth, with a mean growth inhibition of 63.8 ± 5.0%. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Chun KH, Lee HY, Hassan K, Khuri F, Hong WK, Lotan R. Implication of protein kinase B/Akt and Bcl-2/Bcl-XL suppression by the farnesyl transferase inhibitor SCH66336 in apoptosis induction in squamous carcinoma cells. Cancer Res. 2003 Aug 15;63(16):4796-800. [2]. Feldkamp MM, Lau N, Roncari L, Guha A. Isotype-specific Ras.GTP-levels predict the efficacy of farnesyl transferase inhibitors against human astrocytomas regardless of Ras mutational status. Cancer Res. 2001 Jun 1;61(11):4425-31. |
|
| Description | Lonafarnib is an inhibitor of farnesyl transferase (FT) with IC50 values of 1.9, 2.8, 5.2 and 10-100 nM for H-Ras, N-Ras, K-Ras and Rheb, respectively. | |||||
| Targets | FT | FT | FT | FT | ||
| IC50 | 1.9 nM (H-Ras) | 2.8 nM (N-Ras) | 5.2 nM (K-Ras) | 10-100 nM (Rheb) | ||
Quality Control & MSDS
- View current batch:
Chemical structure