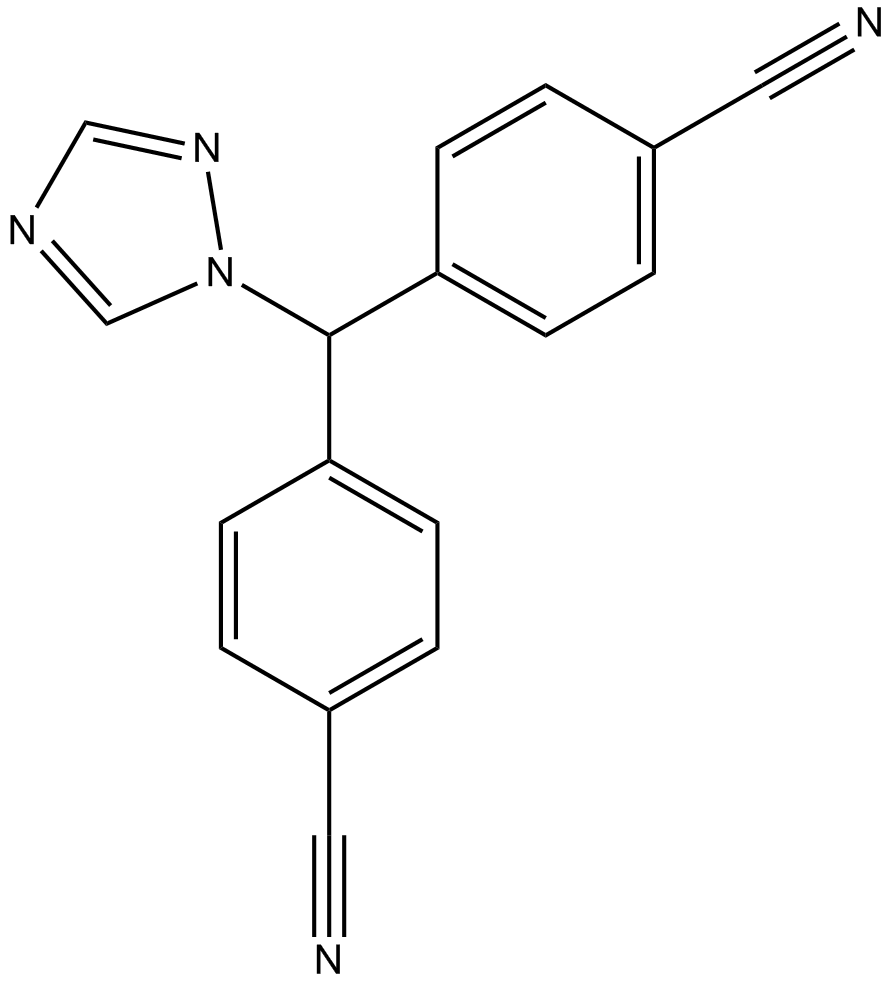
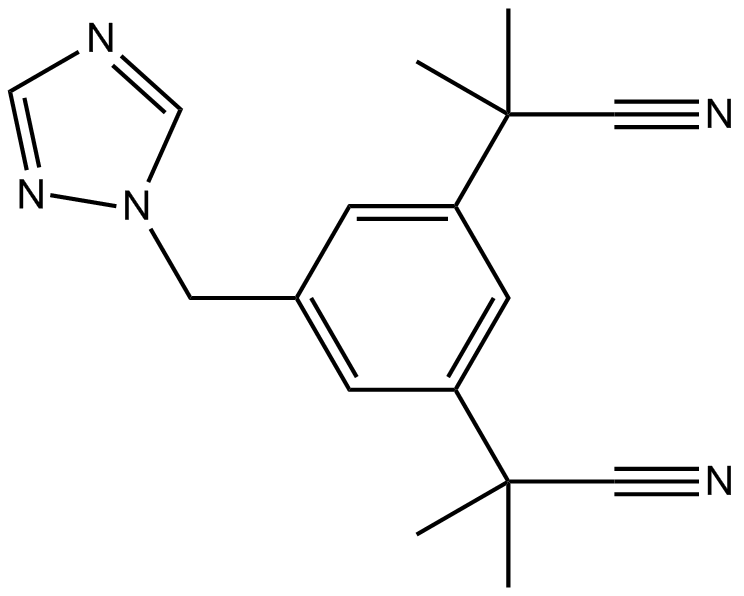
Letrozole
Letrozole is a novel and potent type (II) aromatase inhibitor with IC50 of 11.5 nM. It belongs to the reversible non-steroidal compound family which can inhibit Arom. However, there is no evidence that it can affect the adrenal steroidogenesis.[1]
Normally, Type II inhibitors have a common feature of heterocyclic azole moiety, which can be binded to the heme–iron in aromatase. It contains a structure of 1,2,4-triazole moieties which can coordinate the heme–iron of cytochrome P450. Meanwhile, benzonitrile substituted letrozole can mimic a unique enzyme structure of the substrate androstenedione.[2]
Letrozole administration can reduce spine synapse and axon outgrowth and it also will decrease the expression of estrogen receptor (ER). While, the synaptic proteins including GAP-43 can impaire the long-termpotentiation.[1] Letrozole is proved to promote FSH release from the hypothalamic pituitary axis by responding to decreased estrogen (E) feedback.[3]
References:
[1] Chen Bian, Yangang Zhao, Qiang Guo, Ying Xiong, Wenqin Cai, Jiqiang Zhang. Aromatase inhibitor letrozole downregulates steroid receptor coactivator-1 in specific brain regions that primarily related to memory, neuroendocrine and integration. The Journal of Steroid Biochemistry and Molecular Biology. May 2014. 141: 37-43.
[2] Hakki Türker Akçay, Riza Bayrak. Computational studies on the anastrozole and letrozole, effective chemotherapy drugs against breast cancer. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 25 March 2014. 122: 142-152.
[3] Lindsay Malloch, Alice Rhoton-Vlasak. An assessment of current clinical attitudes toward letrozole use in reproductive endocrinology practices. Fertility and Sterility. December 2013. 100(6): 1740-1744.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 285.3 |
| Cas No. | 112809-51-5 |
| Formula | C17H11N5 |
| Solubility | insoluble in EtOH; insoluble in H2O; ≥14.265 mg/mL in DMSO |
| Chemical Name | 4,4'-((1H-1,2,4-triazol-1-yl)methylene)dibenzonitrile |
| SDF | Download SDF |
| Canonical SMILES | N#CC1=CC=C(C(N2N=CN=C2)C3=CC=C(C#N)C=C3)C=C1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure