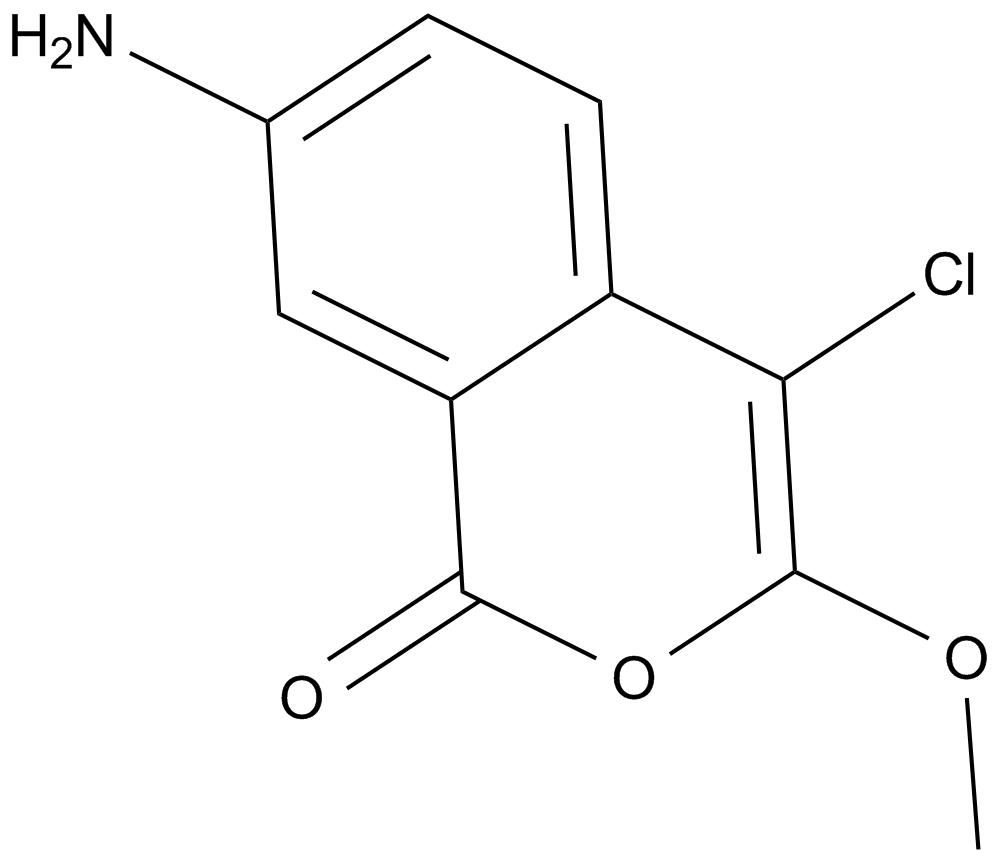
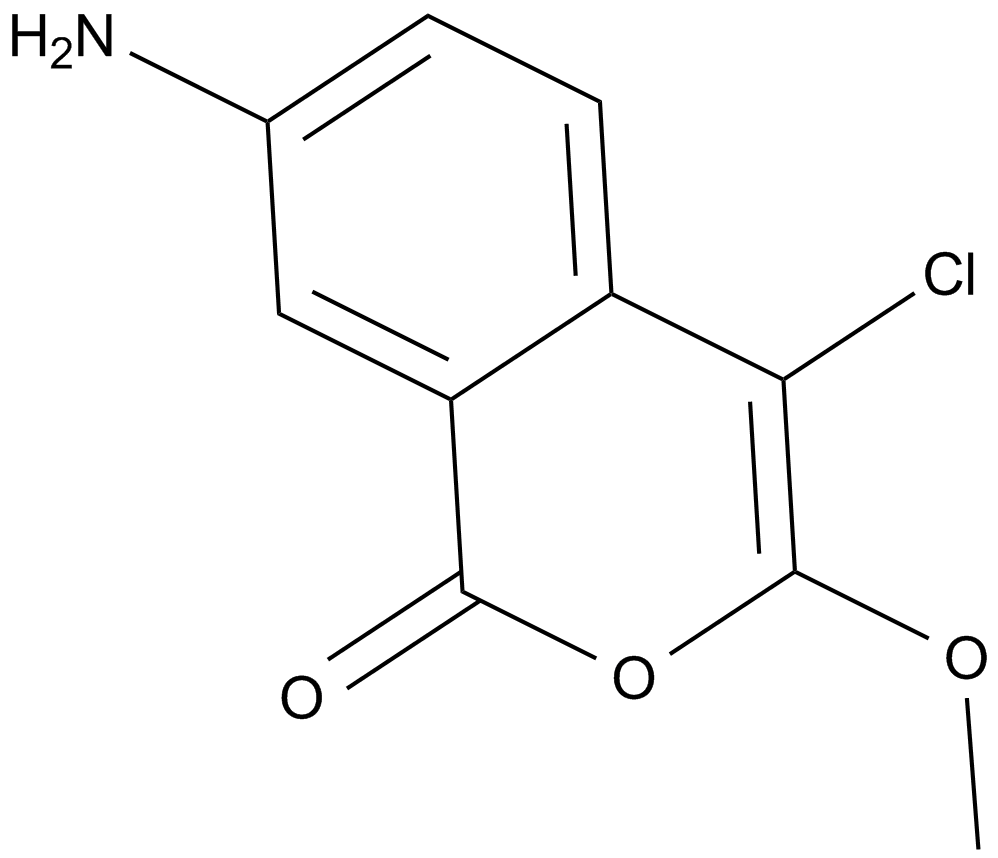
JLK 6
JLK 6, an isocoumarin, is a selective inhibitor of γ-secretase, with an IC50 value between 10 μM-1 mM [1, 2].
The enzyme γ-secretase catalyzes the cleavage of β-Amyloid precursor protein (βAPP) to produce Amyloid β-peptide (Aβ). Aβ is a part of the plaque present in the brain of patients with Alzheimer’s disease. γ-secretase also targets other substrates like Notch. Notch is a transmembrane protein which is involved in important functions during different stages in development, both embryonic and adulthood [1].
HEK293 cells were used. In these cells, wild-type βAPP was overexpressed (962 fmol/mL in 35-mm wells). JLK6 markedly reduced Aβ secreted from these cells. Interestingly, JLK6 potentiated the recovery of two fragments. Immunological characterization indicated that one fragment was labelled with a specific antibody against the Asp1 residue of Aβ. JLK6 also inhibited the Aβ recovery from cells overexpressing Swedish-mutant βAPP to a similar extent [2].
In the zebrafish embryo, JLK isocoumarin inhibitors did not change the Notch pathway responsible for somitogenesis. Unlike other γ-secretase inhibitors, these agents did not affect E-cadherin processing. JLKs did not inhibit α-secretase, β-site APP cleaving enzymes (BACE) 1 and BACE2, GSK3β kinase and proteasome. JLK inhibitors prevented Aβ production without inducing unwanted cleavages of other proteins [1].
References:
[1]. Petit A, Pasini A, Alves Da Costa C, et al. JLK isocoumarin inhibitors: Selective γ-secretase inhibitors that do not interfere with notch pathway in vitro or in vivo. Journal of neuroscience research, 2003, 74(3): 370-377.
[2]. Petit A, Bihel F, da Costa CA, et al. New protease inhibitors prevent γ-secretase-mediated production of Aβ40/42 without affecting Notch cleavage. Nature cell biology, 2001, 3(5): 507-511.
| Physical Appearance | Yellow solid |
| Storage | Store at RT |
| M.Wt | 225.63 |
| Cas No. | 62252-26-0 |
| Formula | C10H8ClNO3 |
| Synonyms | 7-Amino-4-chloro-3-methoxy-1H-2-benzopyran |
| Solubility | <22.56mg/ml in DMSO; <5.64mg/ml in ethanol |
| Chemical Name | 7-amino-4-chloro-3-methoxy-1H-isochromen-1-one |
| SDF | Download SDF |
| Canonical SMILES | COC1=C(Cl)C2=CC=C(N)C=C2C(O1)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure