Itraconazole
Itraconazole (CAS: 84625-61-6) is a triazole-based antifungal compound that functions primarily by inhibiting cytochrome P450 enzymes, particularly CYP3A4. As both a substrate and inhibitor of CYP3A4, itraconazole undergoes oxidative metabolism into hydroxylated, keto-, and N-dealkylated derivatives, all with demonstrated inhibitory activities comparable or superior to the parent compound. In addition to antifungal effects, itraconazole inhibits the hedgehog signaling pathway and angiogenesis. Due to its affinity for mammalian CYP-P450 enzymes, itraconazole is frequently employed in drug interaction studies assessing CYP3A-mediated metabolism.
- 1. Wenying Cai, Junhao Huang, et al. "Combinatory Effect of ALA-PDT and Itraconazole In the Treatment of Cutaneous protothecosis." Photodiagnosis Photodyn Ther. 2024 Sep 13:49:104332 PMID: 39278300
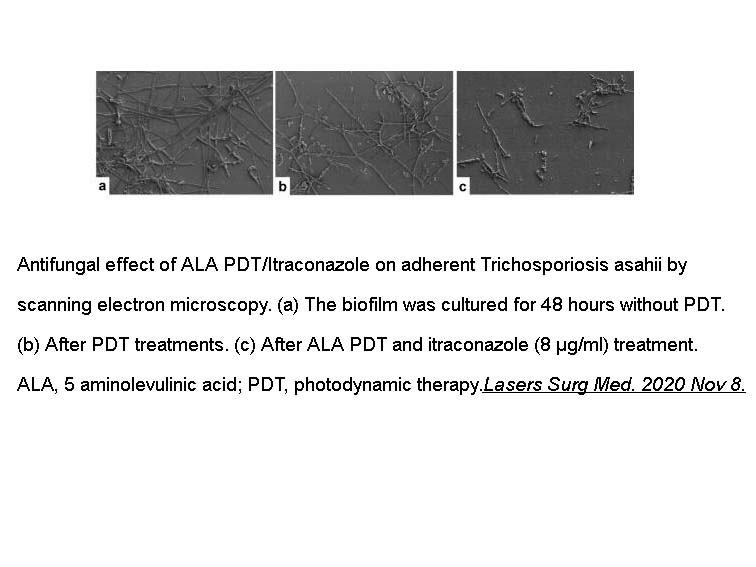
- 2. Yu Lan, Sha Lu, et al. "Combinatory Effect of ALA‐PDT and Itraconazole Treatment for Trichosporon asahii." Lasers Surg Med. 2020 Nov 8. PMID:33164224
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 705.63 |
| Cas No. | 84625-61-6 |
| Formula | C35H38Cl2N8O4 |
| Solubility | insoluble in EtOH; insoluble in H2O; ≥8.83 mg/mL in DMSO |
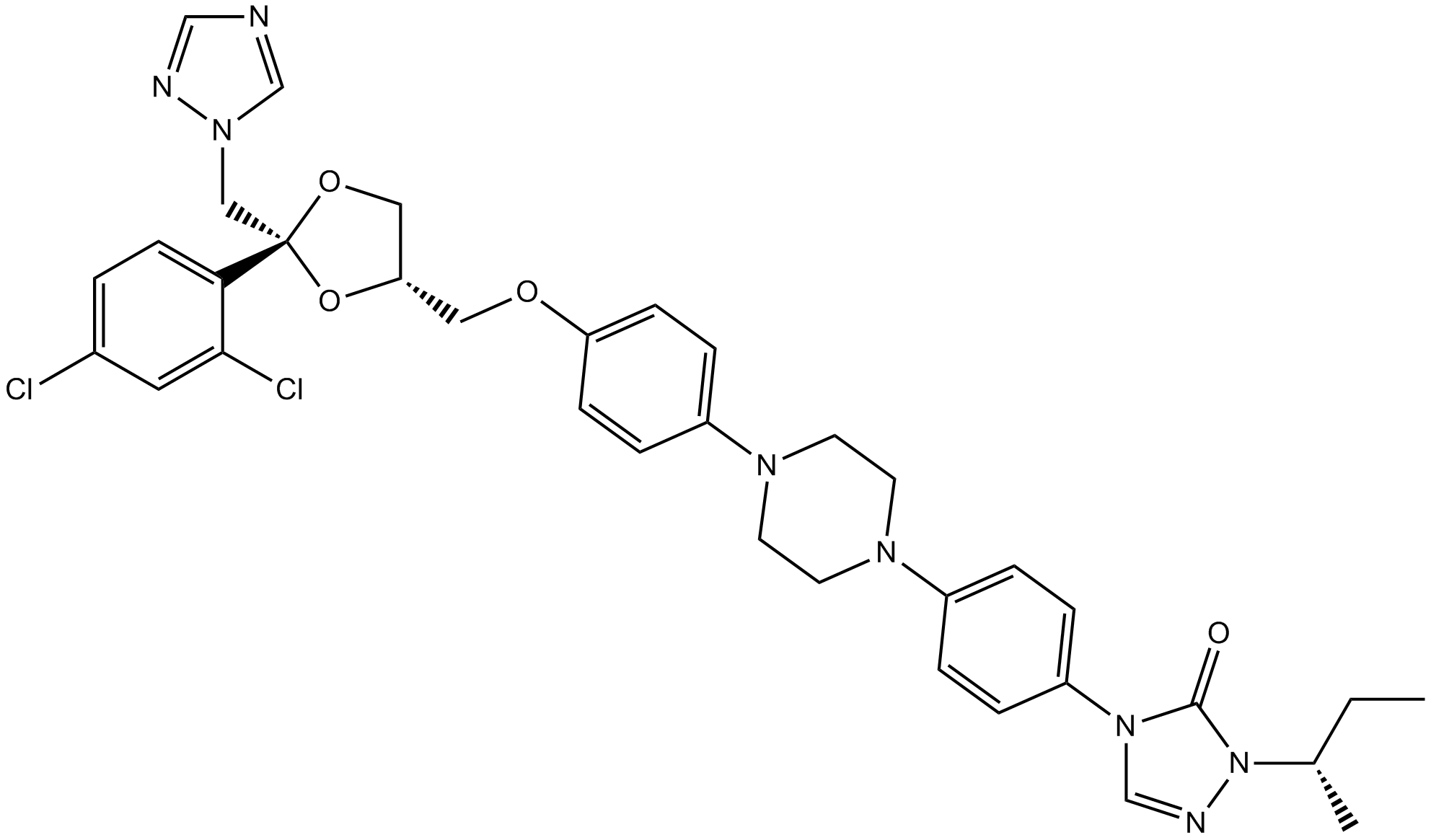
| Chemical Name | 2-butan-2-yl-4-[4-[4-[4-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-1,2,4-triazol-3-one |
| SDF | Download SDF |
| Canonical SMILES | CCC(C)N1C(=O)N(C=N1)C2=CC=C(C=C2)N3CCN(CC3)C4=CC=C(C=C4)OCC5COC(O5)(CN6C=NC=N6)C7=C(C=C(C=C7)Cl)Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
C. glabrata, C. kefyr |
|
Preparation method |
The solubility of this compound in DMSO is > 8.8mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0.016 μg/ml |
|
Applications |
Itraconazole showed antifungal activity against 206 isolates of C. glabrata. For 144 isolates, two or more replicate test results were recovered from the database. In five replicate tests with two bioassay strains of C. kefyr (SA and ATCC 46764), the IC50 of itraconazole was 0.016 mg/L. |
| Animal experiment [2]: | |
|
Animal models |
CD1 mice model of disseminated candidiasis |
|
Dosage form |
5 mg/kg, twice daily for 5 days |
|
Application |
Treatment with ITZ led to lower numbers of CFU per gram of kidney. The survival rates for mice inoculated with strain Sr and isolate B were 7 of 10 in mice treated with ITZ. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Odds F C, Bossche H V. Antifungal activity of itraconazole compared with hydroxy-itraconazole in vitro[J]. Journal of Antimicrobial Chemotherapy, 2000, 45(3): 371-373. [2]. Valentin A, Le Guennec R, Rodriguez E, et al. Comparative resistance of Candida albicans clinical isolates to fluconazole and itraconazole in vitro and in vivo in a murine model[J]. Antimicrobial agents and chemotherapy, 1996, 40(6): 1342-1345. | |
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data