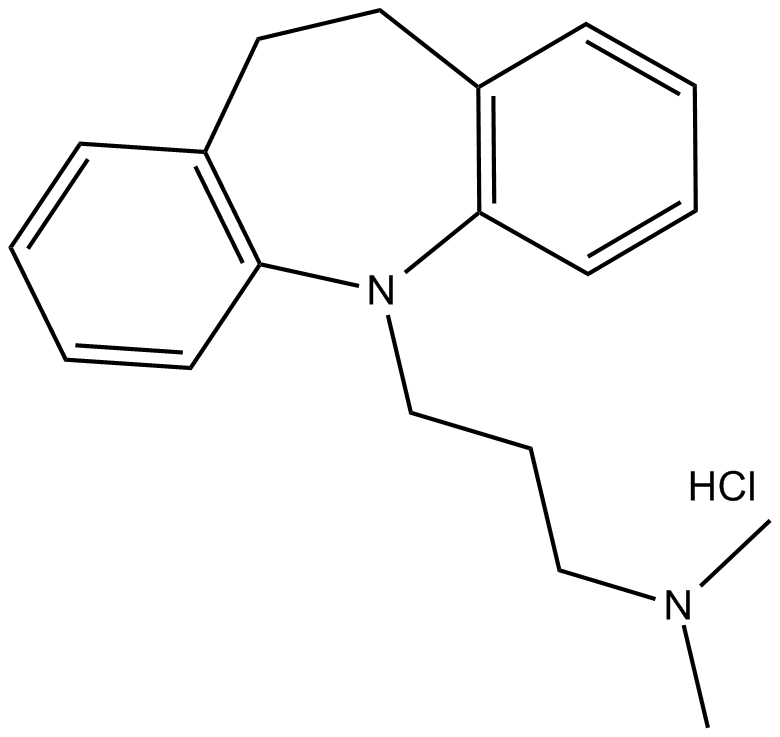
Imipramine (hydrochloride)
Imipramine (hydrochloride) is a tricyclic antidepressant and is mainly used in the treatment of major depression and enuresis [1].
Antidepressants are antagonists of many neurotransmitter receptors in human brain [3].
Imipramine is the first tricyclic antidepressant that acts mainly as an inhibitor of serotonin and norepinephrine transporters [2]. In radioligand binding assays, imipramine inhibited serotonin and norepinephrine transporters with KD values of 1.4 and 37 nM, respectively [2]. Imipramine is also inhibited histamine H1 receptor, muscarinic acetylcholine receptor and α1-adrenergic receptor with Kd values of 37, 46, and 32 nM, respectively [4].
In rodents, imipramine abolished the depressive syndrome produced by the acute administration of reserpine. Imipramine also possessed central anticholinergic activity and attenuate the activity of the centrally acting muscarinic agents tremorine and oxotremorine. Imipramine inhibited the presynaptic uptake of NA and 5-HT, and relatively weak against DA [1].
References:
[1]. Spencer PS. Review of the pharmacology of existing antidepressants. Br J Clin Pharmacol. 1977;4Suppl 2:57S-68S.
[2]. Tatsumi M, Groshan K, Blakely RD, et al. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997 Dec 11;340(2-3):249-58.
[3]. Cusack B, Nelson A, Richelson E. Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65.
| Storage | Store at -20°C |
| M.Wt | 316.9 |
| Cas No. | 113-52-0 |
| Formula | C19H24N2·HCl |
| Synonyms | Melipramine,Tofranil |
| Solubility | ≥12.5 mg/mL in DMSO; ≥22.3 mg/mL in H2O; ≥22.9 mg/mL in EtOH |
| Chemical Name | 10,11-dihydro-N,N-dimethyl-5H-dibenz[b,f]azepine-5-propanamine, monohydrochloride |
| SDF | Download SDF |
| Canonical SMILES | CN(C)CCCN(c1c(CC2)cccc1)c1c2cccc1.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure