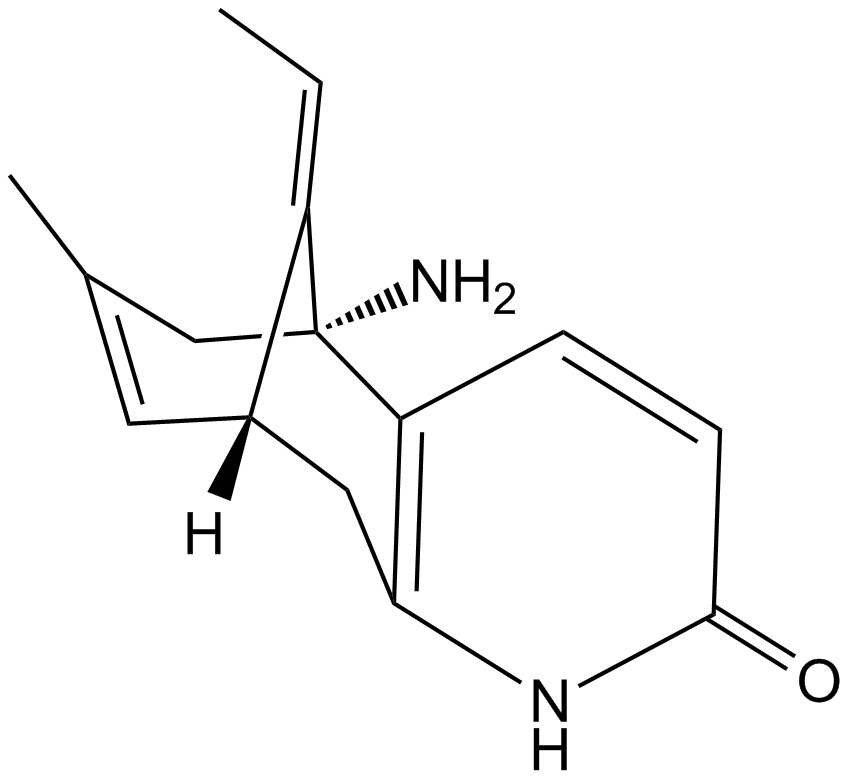
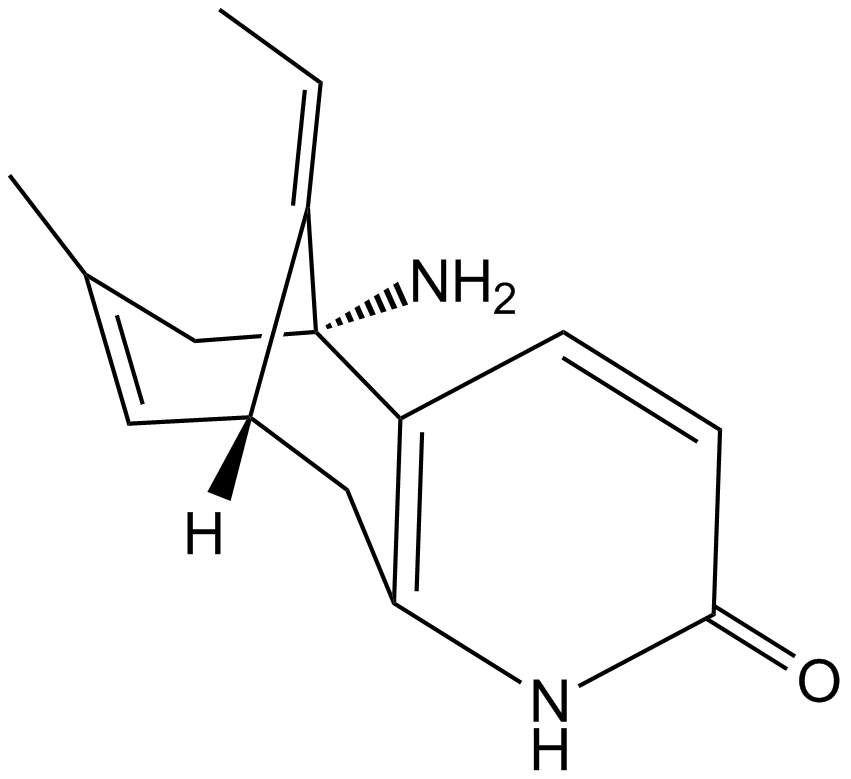
(-)-Huperzine A
(−)-Huperzine A (HupA) is an acetylcholinesterase (AChE) inhibitor with an IC50 value of 82 nmol/L [1] and acts as an antagonist of the N-methyl-d-aspartate (NMDA) receptor [2].
AChE is the key brain enzyme responsible for the rapid degradation of the neurotransmitter acetylcholine. AChE inhibitors are probably useful in the amelioration of the Alzheimer’s symptomatology [3].
It was found that NMDA markedly reduced AChE activities [4]. In rat dissociated hippocampal neurons, HupA inhibited the NMDA-induced current. In neurons, 100 µM HupA, NMDA-induced currents were 55.7 ± 4.9% of the control values. The binding molecular ratio of NMDA receptor: HupA is 1:1. The inhibition of NMDA receptor by HupA is not competitive [5]. HupA significantly increased the phosphorylation levels of both glycogen synthase kinase (GSK)-3α protein and GSK-3β protein in APPsw-overexpressing cells [2]. Activated GSK-3 consequently decreased acetylcholine (ACh) level in the striatum [6].
Treated with doses of (−)-huperzine A, AChE−/− mice showed no toxic symptoms and had normal levels of AChE. This demonstrated the specificity of (−)-huperzine A as an inhibitor of AChE at the dose used in vivo [7]. In rat whole brain, oral administration of HupA at a dose of 1.5 μmol/kg (3.6 mg/kg) obtained a maximum inhibition of AChE at 60 min and this maximum inhibition was maintained for 360 min [8].
References:
[1]. MA Xiao-Chao, XIN Jian, WANG Hai-Xue, et al. Acute effects of huperzine A and tacrine on rat liver. Acta Pharmacol ogica Sinica, 2003, 24(3):247-250.
[2]. Zhong Ming Qian and Ya Ke. Huperzine A: is it an effective disease-modifying drug for Alzheimer's disease? Frontiers in Aging Neuroscience, 2014, 6:216.
[3]. V. Rajendran, Suo-Bao Rong, Ashima Saxena, et al. Synthesis of a hybrid analog of the acetylcholinesterase inhibitors huperzine A and huperzine B. Tetrahedron Letters, 2001, 42: 5359-5361.
[4]. J. R. Delfs, D. M. Saroff, Y. Nishida, et al. Effects of NMDA and its antagonists on ventral horn cholinergic neurons in organotypic roller tube spinal cord cultures. J. Neural Transm., 1997, 104(1):31-51.
[5]. J. M. Zhang and G. Y. Hu. Huperzine A, a nootropic alkaloid, inhibits N-methyl-D-aspartate-induced current in rat dissociated hippocampal neurons. Neuroscience, 2001, 105(3):663-9.
[6]. L. Zhao, C. B. Chu, J. F. Li, et al. Glycogen synthase kinase-3 reduces acetylcholine level in striatum via disturbing cellular distribution of choline acetyltransferase in cholinergic interneurons in rats. Neuroscience, 2013, 255:203-11.
[7]. Ellen G. Duysen, Bin Li, Sultan Darvesh, et al. Sensitivity of butyrylcholinesterase knockout mice to (−)-huperzine A and donepezil suggests humans with butyrylcholinesterase deficiency may not tolerate these Alzheimer’s disease drugs and indicates butyrylcholinesterase function in neurotransmission. Toxicology, 2007, 233:60-69.
[8]. Rui Wang, Han Yan and Xi-can Tang. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacologica Sinica, 2006, 27:1-26.
- 1. Liqun Lin, Cheng Li, et al. "Phyllosphere mycobiome in two Lycopodiaceae plant species: unraveling potential HupA-producing fungi and fungal interactions." Front Plant Sci. 2025 Mar 14:16:1426540 PMID: 40161220
- 2. Ryan S Nett, Yaereen Dho, et al. "Plant carbonic anhydrase-like enzymes in neuroactive alkaloid biosynthesis." Nature. 2023 Dec;624(7990):182-191. PMID: 37938780
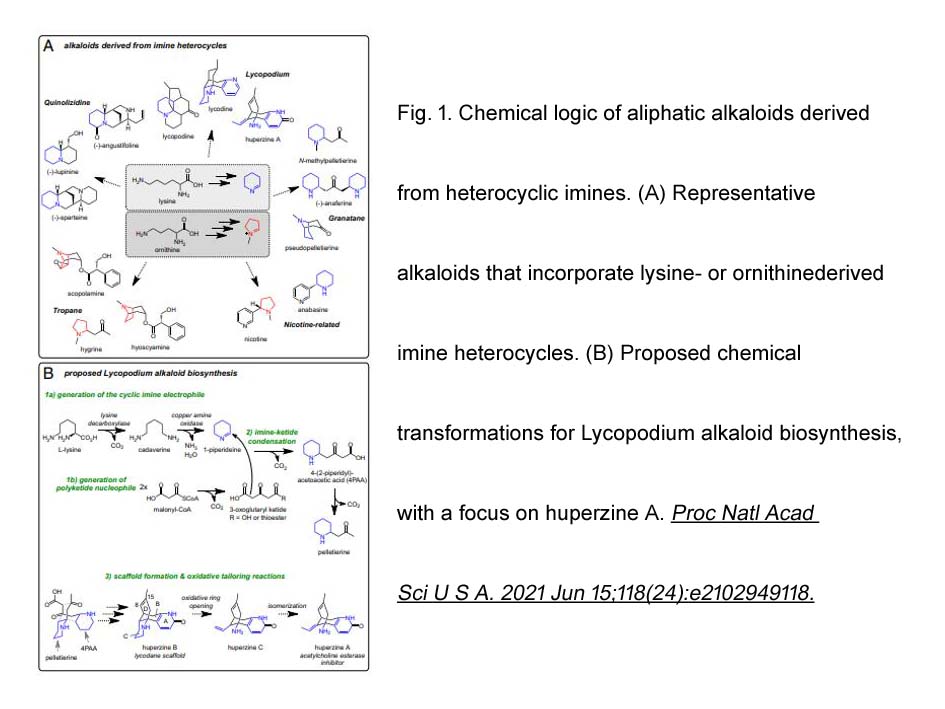
- 3. Ryan S. Nett, Yaereen Dho, et al. "A metabolic regulon reveals early and late acting enzymes in neuroactive Lycopodium alkaloid biosynthesis." Proc Natl Acad Sci U S A. 2021 Jun 15;118(24):e2102949118. PMID:34112718
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 242.3 |
| Cas No. | 102518-79-6 |
| Formula | C15H18N2O |
| Synonyms | Huperzine A, |
| Solubility | insoluble in H2O; ≥12.12 mg/mL in DMSO; ≥23.13 mg/mL in EtOH |
| Chemical Name | (5R,9R,E)-5-amino-11-ethylidene-7-methyl-5,6,9,10-tetrahydro-5,9-methanocycloocta[b]pyridin-2(1H)-one |
| SDF | Download SDF |
| Canonical SMILES | C/C=C(\[C@H](CC(N1)=C2C=CC1=O)C=C(C)C1)/[C@@]21N |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Description | (-)-Huperzine A is a potent, highly specific and reversible inhibitor of acetylcholinesterase (AChE) with Ki of 7 nM, exhibiting 200-fold more selectivity for G4 AChE over G1 AChE. | |||||
| Targets | Acetylcholinesterase (G4 form) | |||||
| IC50 | 7 nM (Ki) | |||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data