HOBt
HOBt (1-Hydroxybenzotriazole; CAS 2592-95-2) is an organic benzotriazole derivative extensively utilized in peptide chemistry. Structurally, it is characterized as a crystalline powder typically containing bound water molecules (approximately 11.7% by weight). Functionally, HOBt serves as a racemization inhibitor during peptide coupling, minimizing epimerization of stereocenters. In peptide synthesis, it participates in the generation of reactive ester intermediates, such as N-hydroxysuccinimide esters. These activated esters react under mild conditions with amino groups, facilitating efficient amide bond formation. Additionally, HOBt enables preparation of amide analogues from carboxylic acids not readily convertible into acyl chlorides, thus expanding synthesis options for antibiotic derivatives and related bioactive molecules.
References:
[1]. Knig W, Geiger R. EineneueMethodezurSynthese von Peptiden: Aktivierung der CarboxylgruppemitDicyclohexylcarbodiimidunterZusatz von 1‐Hydroxy‐benzotriazolen[J]. ChemischeBerichte, 1970, 103(3): 788-798.
[2]. Myers A G, Yang B H, Chen H. Transformation of Pseudoephedrine Amides into Highly Enantiomerically Enriched Aldehydes, Alcohols, and Ketones[J]. Organic Syntheses, 2000: 29-29.
[3]. owicki D, Huczyński A, Ratajczak-Sitarz M, et al. Structural and antimicrobial studies of a new N-phenylamide of monensin A complex with sodium chloride[J]. Journal of Molecular Structure, 2009, 923(1): 53-59.
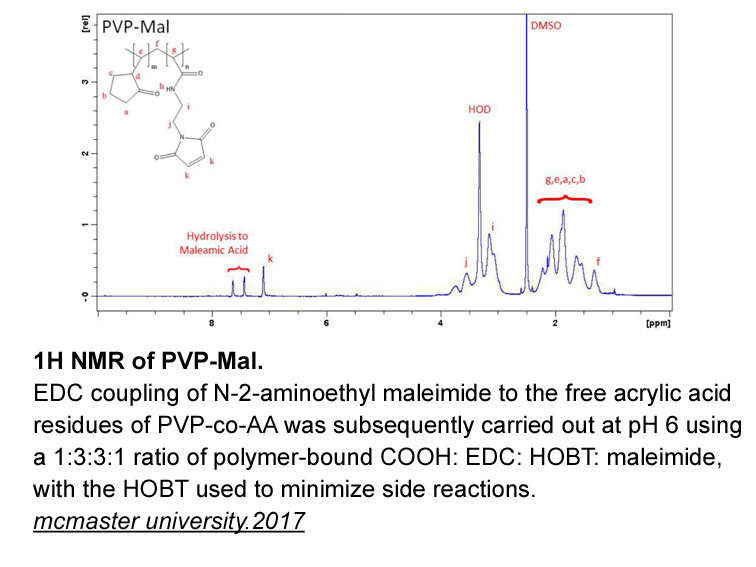
- 1. Sachin B. Baravkar, Yan Lu, et al. "Rationally Designed Pentapeptide Analogs of Aβ19–23 Fragment as Potent Inhibitors of Aβ42 Aggregation." Molecules. 2025 May 7;30(9):2071 PMID: 40363876
- 2. Song Hong, Sachin B. Baravkar, et al. "Molecular Modification of Queen Bee Acid and 10-Hydroxydecanoic Acid with Specific Tripeptides: Rational Design, Organic Synthesis, and Assessment for Prohealing and Antimicrobial Hydrogel Properties." Molecules. 2025 Jan 30;30(3):615 PMID: 39942719
- 3. Herman, Noah. "The Electrostatic Co-Assembly of Camptothecin and Rhodamine B Containing Dipeptides." 2024, Master of Science, Ohio State University, Chemistry.
- 4. Song Hong, et al. "Development of a Novel Covalently Bonded Conjugate of Caprylic Acid Tripeptide (Isoleucine-Leucine-Aspartic Acid) for Wound-Compatible and Injectable Hydrogel to Accelerate Healing." Biomolecules. 2024 Jan 11;14(1):94. PMID: 38254694
- 5. Ching-Hsin Huang, Edwin Chang, et al. "Tumor protease-activated theranostic nanoparticles for MRI-guided glioblastoma therapy." Theranostics. 2023 Mar 13;13(6):1745-1758. PMID: 37064879
- 6. Megan S Michie, Baogang Xu, et al. "Side-chain modification of collagen-targeting peptide prevents dye aggregation for improved molecular imaging of arthritic joints." J Photochem Photobiol A Chem. 2022 Feb 1:424:113624. PMID: 36406204
- 7. Izabela Maluch, Justyna Grzymska, et al. "Evaluation of the effects of phosphorylation of synthetic peptide substrates on their cleavage by caspase-3 and-7." Biochem J. 2021 Jun 25;478(12):2233-2245. PMID: 34037204
- 8. Rafiei A, Schriemer DC. "A microtubule crosslinking protocol for integrative structural modeling activities." Anal Biochem. 2019 Sep 6:113416. PMID: 31499019
- 9. Mengmeng Feng. "Improving the bioavailability of collagen-derived peptides: studies in cell culture models." University of Alberta. 2019.
- 10. Gilbert T, Alsop RJ, et al. "Nanostructure of Fully Injectable Hydrazone-Thiosuccinimide Interpenetrating Polymer Network Hydrogels Assessed by Small-Angle Neutron Scattering and dSTORM Single-Molecule Fluorescence Microscopy." ACS Appl Mater Interfaces. 2017 Nov 27. PMID: 29131571
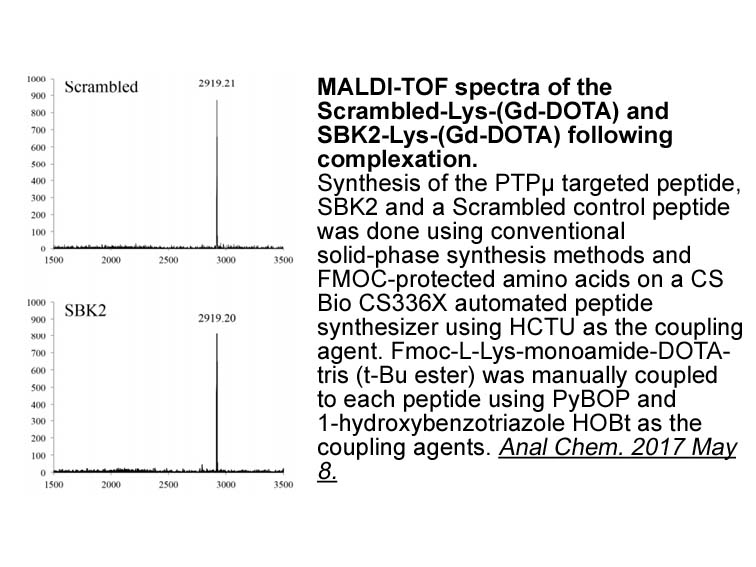
- 11. Johansen ML, Gao Y, et al. "Quantitative Molecular Imaging with a Single Gd-Based Contrast Agent Reveals Specific Tumor Binding and Retention in Vivo." Anal Chem. 2017 Jun 6;89(11):5932-5939. PMID: 28481080
- 12. Gilbert, Trevor. "Injectable Interpenetrating Network Hydrogels for Biomedical Applications." mcmaster university.2017.
- 13. Herrmann, Kelsey, et al. "Molecular Imaging of Tumors Using a Quantitative T1 Mapping Technique via Magnetic Resonance Imaging." Diagnostics 5.3 (2015): 318-332.
| Physical Appearance | A solid |
| Storage | Desiccate at -20°C |
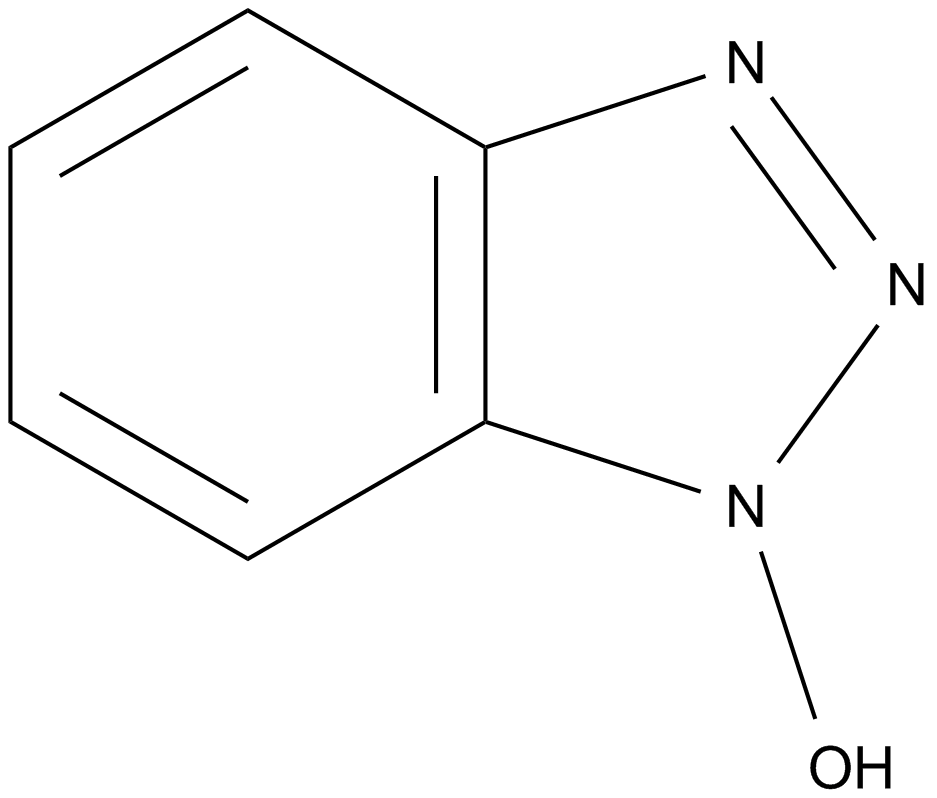
| M.Wt | 135.1 |
| Cas No. | 2592-95-2 |
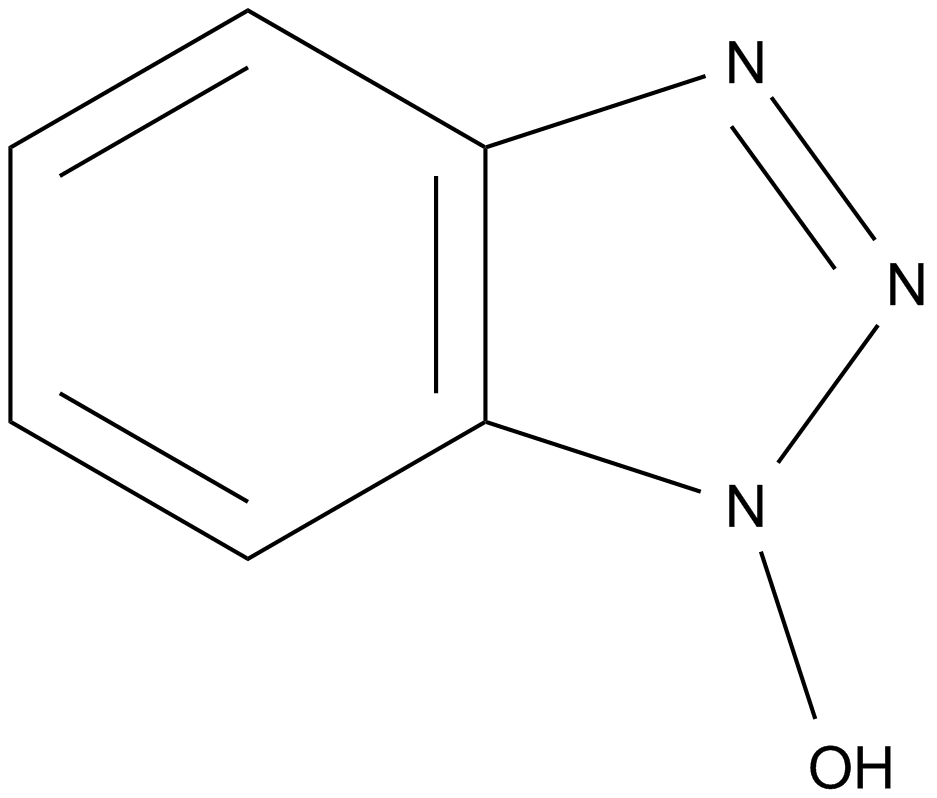
| Formula | C6H5N3O |
| Solubility | ≥22.4 mg/mL in EtOH with ultrasonic; ≥4.09 mg/mL in H2O with ultrasonic; ≥6.76 mg/mL in DMSO |
| Chemical Name | 1-hydroxybenzotriazole |
| SDF | Download SDF |
| Canonical SMILES | O[n]1nnc2c1cccc2 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Description | HOBt is an inhibitor of racemization for peptide synthesis. | |||||
| Targets | racemization | |||||
| IC50 | ||||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data