Geldanamycin
Geldanamycin, a crystalline antimicrobial compound derived from the culture filtrates of Streptomyces hygroscopicus var. geldanus var. nova., is a potent and specific inhibitor of heat shock protein 90 (Hsp90) that specifically binds to the unique ATP binding pocket of Hsp90 in a stable and pharmacologically specific manner. As an antimicrobial agent, geldanamycin exhibits moderate activity against protozoa, bacteria and fungi as well as parasite Syphacia oblevata and cell cultures of L-1210 and KB. Recent studies have shown that geldanamycin also inhibits the function of glucocorticoid receptor and endothelium-dependent relaxation of the rat aorta, mesentery and middle artery.
Reference
Bucci M, Roviezzo F, Cicala C, Sessa WC, Cirino G. Geldanamycin, an inhibitor of heat shock protein 90 (Hsp90) mediated signal transduction has anti-inflammatory effects and interacts with glucocorticoid receptor in vivo. Br J Pharmacol. 2000; 131(1): 13-16.
DeBoer C, Meulman PA, Wnuk RJ, Peterson DH. Geldanamycin, a new antibiotic. J Antibiot (Tokyo). 1970; 23(9):442-447.
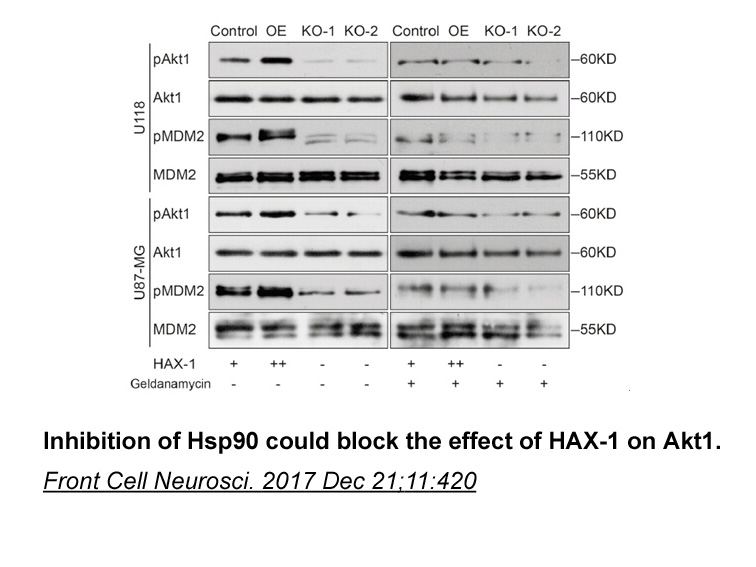
- 1. Deng X, Song L, et al. "HAX-1 Protects Glioblastoma Cells from Apoptosis through the Akt1 Pathway." Front Cell Neurosci. 2017 Dec 21;11:420. PMID:29311840
- 2. Guo XB, Deng X, et al. "Hematopoietic Substrate-1-Associated Protein X-1 Regulates the Proliferation and Apoptosis of Endothelial Progenitor Cells Through Akt Pathway Modulation." Stem Cells. 2018 Mar;36(3):406-419. PMID:29139175
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 560.6 |
| Cas No. | 30562-34-6 |
| Formula | C29H40N2O9 |
| Solubility | insoluble in EtOH; insoluble in H2O; ≥16.9 mg/mL in DMSO |
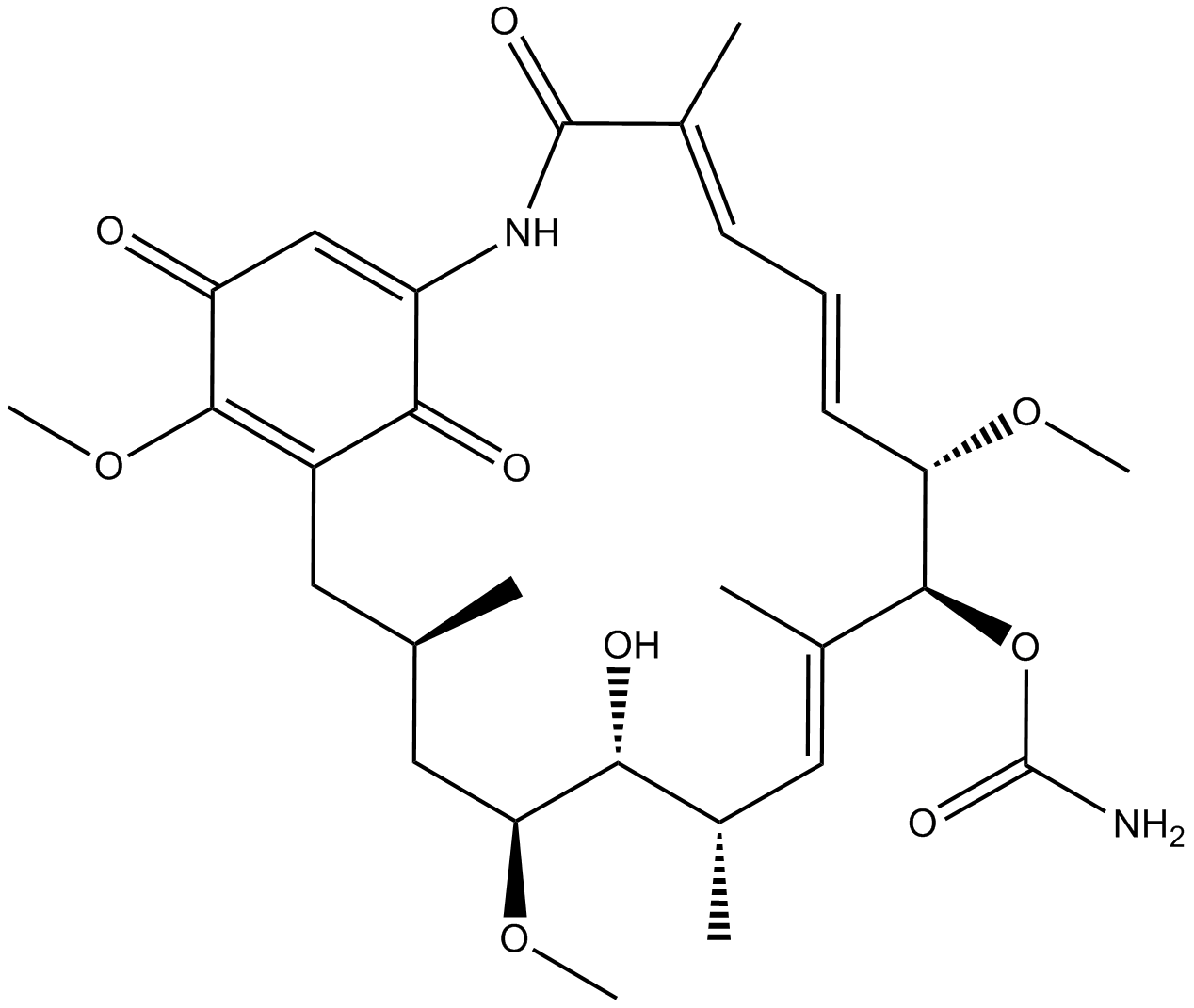
| Chemical Name | [(3R,5S,6R,7S,8E,10S,11S,12Z,14E)-6-hydroxy-5,11,21-trimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate |
| SDF | Download SDF |
| Canonical SMILES | COC(C(C=C1N2)=O)=C(C1=O)C[C@@H](C)C[C@H](OC)[C@H](O)[C@@H](C)/C=C(C)/[C@H](OC(N)=O)[C@@H](OC)/C=C/C=C(C)/C2=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Isothermal titration calorimetry (ITC) of nucelotide binding |
The titration experiments were performed using the MSC system. In each experiment, 16 aliquots of 15 μL of Geldanamycin (300 μM in 1% DMSO) were injected into 1.3 mL of protein (31 μM in 20 mM Tris-HCl, pH 7.5, 1 mM EDTA) at 25°C, and the resulting data were fit after subtracting the heats of dilution. Heats of dilution were determined in separate experiments from addition of Geldanamycin into buffer and buffer into protein. No evidence for binding of DMSO in the nucleotide binding site was observed. Titration data were fit using a nonlinear least-squares curve-fitting algorithm with three floating variables: stoichiometry, binding constant (Kb = 1/Kd), and change of enthalpy of interaction (ΔH°). The dissociation constant estimated for Geldanamycin binding to intact yeast Hsp90 was 1.22 μM, and for binding to Hsp90 N-terminal domain was 0.78 μM. No meaningful heat was observed with binding to the C-terminal fragment. |
| Cell experiment [2]: | |
|
Cell lines |
A2780 cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reaction Conditions |
0.001 ~ 10 μM; 3 hrs |
|
Applications |
In human ovarian cell line A2780, Geldanamycin caused a dose-dependent G2 arrest and reversible inhibiton of entry into the S phase. |
| Animal experiment [3]: | |
|
Animal models |
Mice bearing FRE/erbB-2 tumors |
|
Dosage form |
50, 100, 200 and 400 mg/kg; i.p.; b.i.d., for 5 days |
|
Applications |
In mice bearing FRE/erbB-2 tumors, Geldanamycin (50 mg/kg) shows 30% inhibition on p185-associated phosphotyrosine levels. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999 Jan 28;42(2):260-6. [2]. McIlwrath AJ, Brunton VG, Brown R. Cell-cycle arrest and p53 accumulation induced by geldanamycin in human ovarian tumour cells. Cancer Chemother Pharmacol. 1996;37(5):423-8. [3]. Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, Moyer JD, DiOrio CI, Barbacci EG, et al. Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogeldanamycin derivatives. J Med Chem. 1995 Sep 15;38(19):3806-12. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
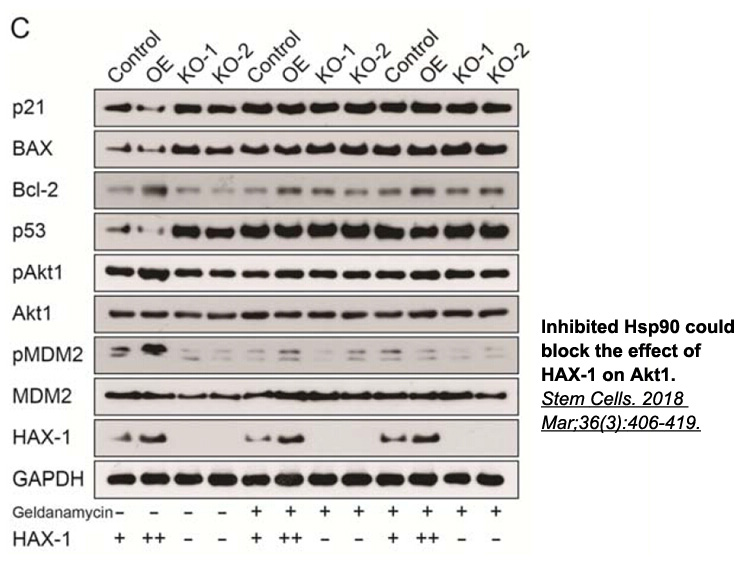
Related Biological Data