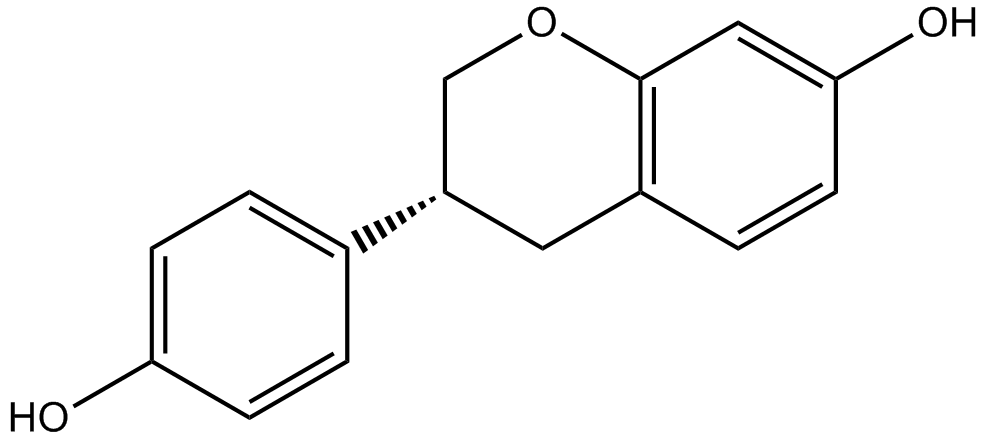
Equol
IC50: Not available.
Equol is an isoflavan produced by intestinal bacteria in response to soy isoflavone intake in human. It shows a wide range of activities including antioxidant activity, anti-inflammation activity and anticancer activity. It is reported that Equol specifically binds to 5α-DHT and has a modest affinity for recombinant estrogen receptor ERβ, which may be responsible for most of Equol’s biological properties. [1]
In vitro: In vitro studies were conducted to measure both the binding affinity of Equol for 5alpha-dihydrotestosterone (5alpha-DHT) and the effects of Equol treatment in human prostate cancer (LNCap) cells. It was found that Equol bound to 5alpha-DHT with maximum and half maxim concentrations of 100 nM and 4.8 nM, respectively. In addition, Equol significantly offset the increases in PSA levels from LNCap cells. [1]
In vivo: An in vivo study was performed to investigate effects of equol on rat prostate weight and circulating levels of sex steroid hormones. 1.0 mg/kg of Equol was injected to Long-Evans rats fed with a low isoflavone diet for 25 days. Findings from this study suggested that Equol significantly decreased rat prostate weights and down-regulated serum levels of 5alpha-DHT. However, this agent did not alter levels of LH, testosterone and estradiol. [1]
Clinical trials: A clinical study on hypercholesterolemic patients demonstrated that, after 4 weeks’ dietary intervention with a soy isoflavone-containing food, brachial artery-mediated vasodilatation in equol-producers was notably higher when compared with that in equol-nonproducers. Similar differential effects between equol-producers and nonproducers were aslo reported in arterial stiffness from a study on postmenopausal women taking tibolone. [2]
References:
[1]Lund TD, Blake C, Bu L, Hamaker AN, Lephart ED. iEquol an isoflavonoid: potential for improved prostate health, in vitro and in vivo evidence. Reprod Biol Endocrin. 2011; 9(4): doi: 10.1186/1477-7827-9-4.
[2]Setchell DR and Clerici C. Equol: Pharmacokinetics and biological actions. J Nutr. 2010 Jul; 140(7): 1363S–8S.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 242.27 |
| Cas No. | 531-95-3 |
| Formula | C15H14O3 |
| Solubility | insoluble in H2O; ≥12.1 mg/mL in DMSO; ≥44.8 mg/mL in EtOH |
| Chemical Name | (3S)-3-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-ol |
| SDF | Download SDF |
| Canonical SMILES | Oc1ccc([C@H](C2)COc3c2ccc(O)c3)cc1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure