Eltanexor (KPT-8602)
Eltanexor (KPT-8602) is a second-generation, orally bioavailable inhibitor of exportin 1 (XPO1), with IC50 values of 20 ~ 211 nM in 10 acute myeloid leukemia (AML) cell lines. XPO1, also known as chromosome maintenance protein 1 (CRM1), is an important nuclear-cytoplasmic exporter in eukaryotes that transports a variety of protein cargoes from the nucleus to the cytoplasm, such as tumor suppressor proteins, cell cycle regulators and apoptosis inducers. Inhibition of XPO1-mediated nuclear export is a promising therapeutic strategy for many cancers, including chronic lymphocytic leukemia (CLL), AML, and aggressive lymphomas.
References:
1. Etchin J, Berezovskaya A, Conway AS, et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia, 2017, 31(1): 143-150.
2. Hing ZA, Fung HY, Ranganathan P, et al. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia, 2016, 30(12): 2364-2372.
- 1. Andrew E Evans, Sahida Afroz, et al. "The XPO1 inhibitor Eltanexor modulates the Wnt/尾-catenin signaling pathway to reduce colorectal cancer tumorigenesis." Cancer Res Commun. 2025 Jul 1;5(7):1140-1154. PMID: 40601866
- 2. Andrew E. Evans, Sahida Afroz, et al. "XPO1 inhibition modulates the Wnt/β-catenin signaling pathway to reduce colorectal cancer tumorigenesis." bioRxiv. November 03, 2024.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 428.29 |
| Cas No. | 1642300-52-4 |
| Formula | C17H10F6N6O |
| Solubility | insoluble in H2O; insoluble in EtOH; ≥44 mg/mL in DMSO |
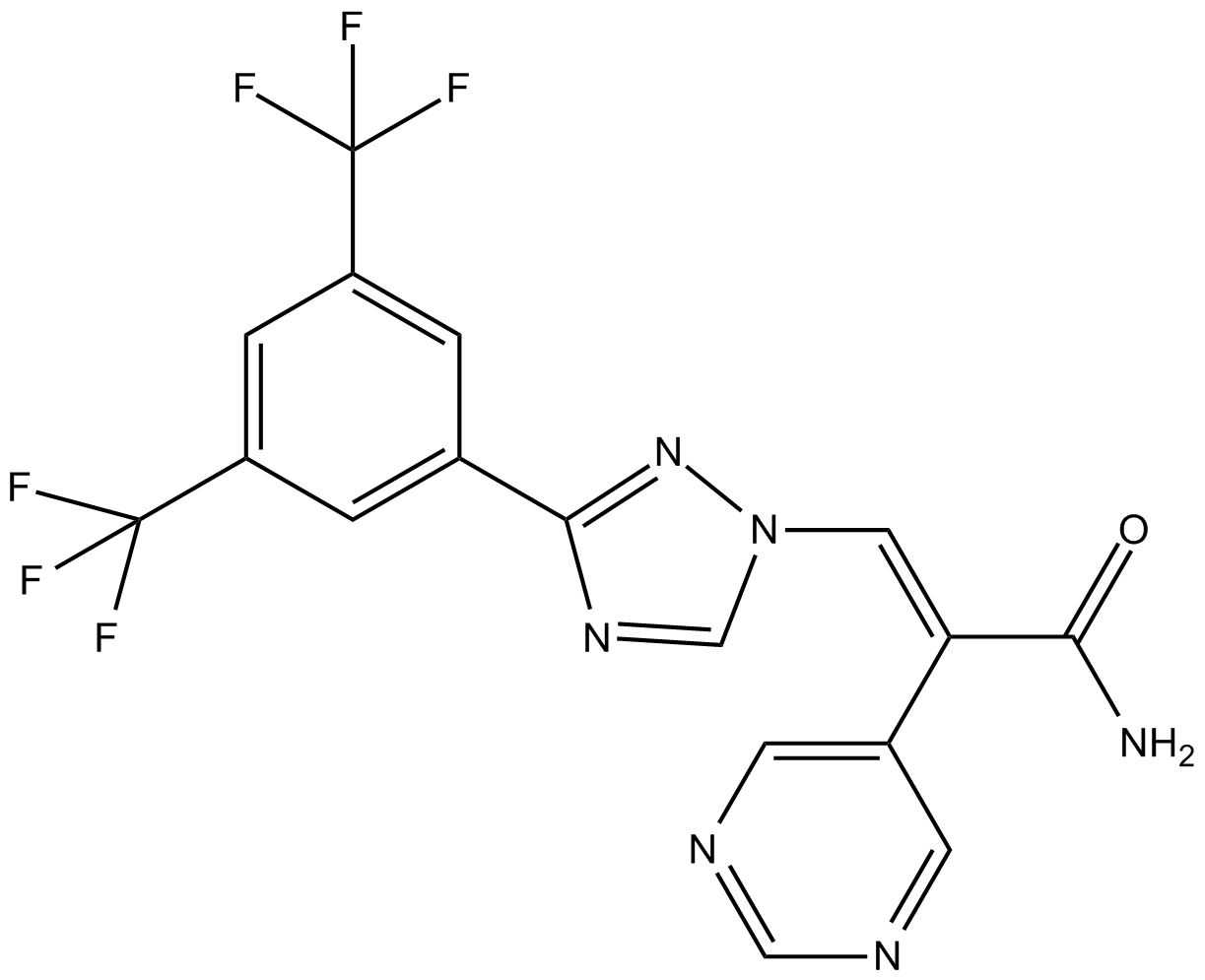
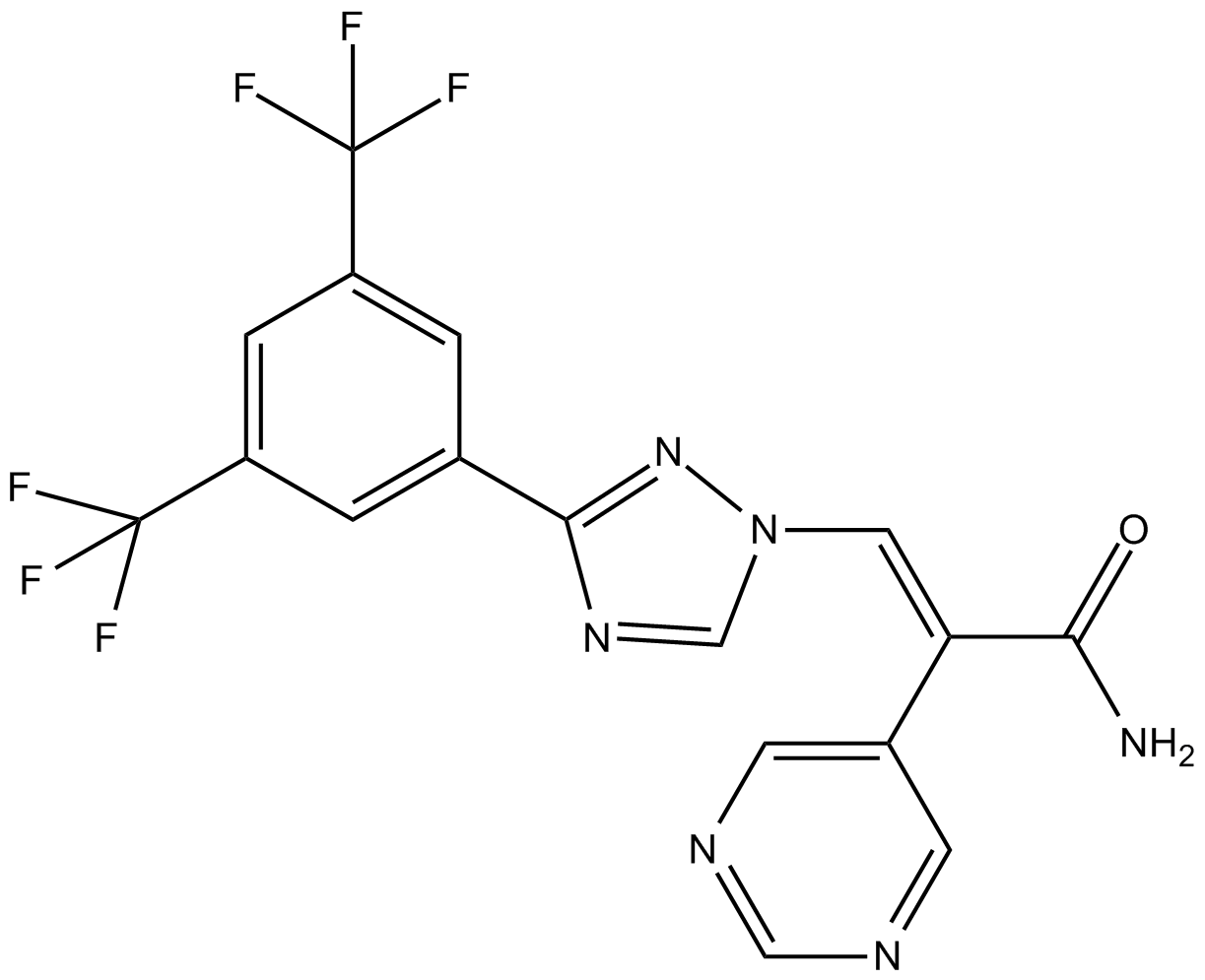
| Chemical Name | (E)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-2-(pyrimidin-5-yl)acrylamide |
| SDF | Download SDF |
| Canonical SMILES | NC(/C(\c1cncnc1)=C/[n]1nc(-c2cc(C(F)(F)F)cc(C(F)(F)F)c2)nc1)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[2] | |
|
Cell lines |
Human primary CLL cells and diffuse large B-cell lymphoma cell lines |
|
Reaction Conditions |
0 ~ 10 μM Eltanexor (KPT-8602) for 48 h incubation |
|
Applications |
Eltanexor (KPT-8602) induced dose-dependent killing of primary CLL cells when compared to vehicle. Eltanexor (KPT-8602) also caused dose-dependent cytotoxicity in the activated B-cell (ABC) subtype and germinal center (GC) subtype of diffuse large B-cell lymphoma cell lines. |
| Animal experiment:[1] | |
|
Animal models |
NOD-SCID-IL2Rcγnull (NSG) mice intravenously injected with various AML cell lines |
|
Dosage form |
15 mg/kg Once daily by oral route for 4 weeks |
|
Applications |
In various AML patient-derived xenograft models, Eltanexor (KPT-8602) exhibited superior anti-leukemic activity and better tolerability than Selinexor, with nearly complete elimination of human AML cells in the normal karyotype AML model. Moreover, KPT-8602 was minimally toxic to normal hematopoietic stem and progenitor cells. |
|
Note |
The technical data provided above is for reference only |
|
References: 1. Etchin J, Berezovskaya A, Conway AS, et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia, 2017, 31(1): 143-150. 2. Hing ZA, Fung HY, Ranganathan P, et al. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia, 2016, 30(12): 2364-2372. |
|
Quality Control & MSDS
- View current batch:
Chemical structure